Abstract
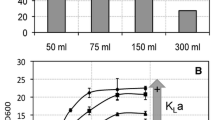
Pseudomonas putida GP01 cells that had accumulated medium-chain-length polyhydroxyalkanoates (PHAMCL) secreted 3-hydroxyoctanoate and 3-hydroxyhexanoate when incubated in alkaline buffers. The release of acids strongly decreased the pH resulting in less efficient secretion of 3HAMCL at neutral pH. To increase the yield of secreted MCL-hydroxyalkanoates, experiments at constant pH in a pH stat apparatus were performed. High acid releasing rates were recorded for the wild type GP01 at pH 9.2 (0.60 mmol acid h−1 g−1 cellular dry weight [cdw]). At more alkaline constant pH values (pH 9.3–11), the initial acid secretion rates were even higher but rapidly decreased by time. When acid secretion of PHA depolymerase mutant GPo500 was tested (pH 9.2), considerably lower rates compared to wild type were recorded (0.18 mmol acid h−1 g−1 cdw). Determination of dissolved oxygen during acid release indicated different respiratoric activity in wild type (low) and mutant (high). Acid release of mutant, but not of the wild type, could be enhanced by aeration. Determination of PHA content of cells after alkaline incubation showed that the wild type had lost most of its accumulated PHA, whereas the PHA content of the depolymerase mutant was not significantly reduced. Considerable amounts of 3HAMCL were secreted by the wild type, but only little 3HAMCL were found for the depolymerase mutant. In summary, 3HAMCL can be more efficiently produced at constant high pH than by incubation without pH control. High PHA depolymerase activity enabled the wild type to compensate for the high external pH by secretion of PHA hydrolysis products, whereas production of protons at aerobic conditions presumably was responsible for the major portion of the observed acid releasing rates in the depolymerase mutant.




Similar content being viewed by others
References
de Eugenio LI et al (2007) Biochemical evidence that phaZ gene encodes a specific intracellular medium chain length polyhydroxyalkanoate depolymerase in Pseudomonas putida KT2442: characterization of a paradigmatic enzyme. J Biol Chem 282:4951–4962
Durst H, Milano M, Kikta E, Conelly S, Grushka E (1975) Phenacyl esters of fatty acids via crown ether catalysts for enhanced ultraviolet detection in liquid chromatography. Anal Chem 47:1797–1801
Foster LJR, Lenz RW, Fuller RC (1994) Quantitative determination of intracellular depolymerase activity in Pseudomonas oleovorans inclusions containing poly-3-hydroxyalkanoates with long alkyl substituents. FEMS Microbiol Lett 118:279–282
Foster LJR, Stuart ES, Tehrani A, Lenz RW, Fuller RC (1996) Intracellular depolymerase and polyhydroxyoctanoate granule integrity in Pseudomonas oleovorans. Int J Biol Macromol 19:177–183
Foster LJR, Lenz RW, Fuller RC (1999) Intracellular depolymerase activity in isolated inclusion bodies containing polyhydroxyalkanoates with long alkyl and functional substituents in the side chain. Int J Biol Macromol 26:187–192
Gebauer B, Jendrossek D (2006) Assay of poly(3-hydroxybutyrate) depolymerase activity and product determination. Appl Environ Microbiol 72:6094–6100
Hippe H, Schlegel HG (1967) Hydrolyse von PHBS durch intracelluläre Depolymerase von Hydrogenomonas H16. Arch Mikrobiol 56:278–299
Huisman GW, Wonink E, Meima R, Kazemier B, Terpstra P, Witholt B (1991) Metabolism of poly(3-hydroxyalkanoates) (PHAs) by Pseudomonas oleovorans. Identification and sequences of genes and function of the encoded proteins in the synthesis and degradation of PHA. J Biol Chem 266:2191–2198
Jendrossek D (2005) Fluorescence microscopical investigation of poly(3-hydroxybutyrate) granule formation in bacteria. Biomacromolecules 6:598–603
Jendrossek D (2007) Peculiarities of PHA granules preparation and PHA depolymerase activity determination. Appl Microbiol Biotechnol (in press)
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56:403–432
Lee SY, Lee Y (2003) Metabolic engineering of Escherichia coli for production of enantiomerically pure (R)-(−)-hydroxycarboxylic acids. Appl Environ Microbiol 69:3421–3426
Lee SY, Lee Y, Wang F (1999) Chiral compounds from bacterial polyesters: sugars to plastics to fine chemicals. Biotechnol Bioeng 65:363–368
Lemoigne M (1925) Etudies sur l’autolyse microbienne acidification par formation d’ucide -oxy-butyrique. Ann Inst Pasteur 39:144–153
Ren Q et al (2005) Bacterial poly(hydroxyalkanoates) as a source of chiral hydroxyalkanoic acids. Biomacromolecules 6:2290–2298
Ruth K, Grubelnik A, Hartmann R, Egli T, Zinn M, Ren Q (2007) Efficient production of (R)-3-hydroxycarboxylic acids by biotechnological conversion of polyhydroxyalkanoates and their purification. Biomacromolecules 8:279–286
Sandoval A et al (2005) Production of 3-hydroxy-n-phenylalkanoic acids by a genetically engineered strain of Pseudomonas putida. Appl Microbiol Biotechnol 67:97–105
Stuart ES, Foster LJR, Lenz RW, Fuller RC (1996) Intracellular depolymerase functionality and location in Pseudomonas oleovorans inclusions containing polyhydroxyoctanoate. Int J Biol Macromol 19:171–176
Vollbrecht D, Schlegel HG (1979) Excretion of metabolites by hydrogen bacteria. III. D(−)-3-hydroxybutanoate. Eur J Appl Microbiol Biotechnol 7:259–266
Acknowledgment
This work was supported by a grant of the Deutsche Forschungsgemeinschaft to D.J. We thank M. Zinn for providing P. putida strains, B. Gebauer for introduction into pH stat apparatus, and O. Vielhauer for support in GC analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Release of acid by P. putida GP01 in pH stat at different pH. 0.25 g (cdw) cells (3 ml) were added to 45 ml of a solution consisting of 10 g/l NaCl, 0.2 g/l MgSO4 × 7 H2O, 0.1 g/l NH4Cl. The pH was adjusted to the value as indicated and kept constant by pH stat (solid line, NaOH consumption; grey line, pH) (PPT 195 kb)
Supplementary Fig. 2
HPLC analysis of products isolated from P. pudia GP01. HPLC chromatogram of isolated hydrolysis products secreted by P. putida GP01 at the end of experiment described in Fig. 2b after derivatisation with BPB. The peaks at 16.2, 17.4 and 21.6 min correspond to unreacted bromophenacylbromide (BPB), 3-hydroxyhexanoate (3HHx) and 3-hydroxyoctanoate (3HO), respectively. The retention times of bromophenacyl derivates differ by up to ±0.3 min depending on platform and batch of buffers used (PPT 48 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Armbruster, W. & Jendrossek, D. Production of medium-chain-length hydroxyalkanoic acids from Pseudomonas putida in pH stat. Appl Microbiol Biotechnol 75, 1047–1053 (2007). https://doi.org/10.1007/s00253-007-0920-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-0920-1