Abstract
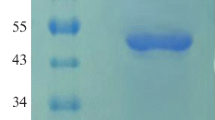
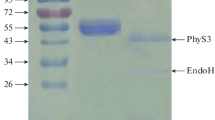
Two novel phytase genes belonging to the histidine acid phosphatase family were cloned from Yersinia rohdei and Y. pestis and expressed in Pichia pastoris. Both the recombinant phytases had high activity at pH 1.5–6.0 (optimum pH 4.5) with an optimum temperature of 55°C. Compared with the major commercial phytases from Aspergillus niger, Escherichia coli, and a potential commercial phytase from Y. intermedia, the Y. rohdei phytase was more resistant to pepsin, retained more activity under gastric conditions, and released more inorganic phosphorus (two to ten times) from soybean meal under simulated gastric conditions. These superior properties suggest that the Y. rohdei phytase is an attractive additive to animal feed. Our study indicated that, in order to better hydrolyze the phytate and release more inorganic phosphorus in the gastric passage, phytase should have high activity and stability, simultaneously, at low pH and high protease concentration.





Similar content being viewed by others
References
Asryants RA, Duszenkova IV, Nagradova NK (1985) Determination of sepharose-bound protein with coomassie brilliant blue G-250. Anal Biochem 151:571–574
Cromwell GL, Stahly TS, Coffey RD, Monegue HJ, Randolph JH (1993) Efficacy of phytase in improving the bioavailability of phosphorus in soybean meal and corn-soybean meal diets for pigs. J Anim Sci 71:1831–1840
Fu D, Huang H, Luo H, Wang Y, Yang P, Meng K, Bai Y, Wu N, Yao B (2008) A highly pH-stable phytase from Yersinia kristeensenii: cloning, expression, and characterization. Enzyme Microb Technol 42:499–505
Garrett JB, Kretz KA, O’Donoghue E, Kerovuo J, Kim W, Barton NR, Hazlewood GP, Short JM, Robertson DE, Gray KA (2004) Enhancing the thermal tolerance and gastric performance of a microbial phytase for use as a phosphate-mobilizing monogastric-feed supplement. Appl Environ Microbiol 70:3041–3046
Haefner S, Knietsch A, Scholten E, Braun J, Lohscheidt M, Zelder O (2005) Biotechnological production and applications of phytases. Appl Microbiol Biotechnol 68:588–597
Han Y, Wilson DB, Lei X (1999) Expression of an Aspergillus niger phytase gene (phyA) in Saccharomyces cerevisiae. Appl Environ Microbiol 65:1915–1918
Haraldsson AK, Veide J, Andlid T, Alminger ML, Sandberg AS (2005) Degradation of phytate by high-phytase Saccharomyces cerevisiae strains during simulated gastrointestinal digestion. J Agric Food Chem 53:5438–5444
Holman WIM (1943) A new technique for the determination of phosphorus by the molybdenum blue method. Biochem J 37:256–259
Huang H, Luo H, Yang P, Meng K, Wang Y, Yuan T, Bai Y, Yao B (2006) A novel phytase with preferable characteristics from Yersinia intermedia. Biochem Biophys Res Commun 350:884–889
Invitrogen (2002) Pichia Expression Kit, a manual of methods for expression of recombinant proteins in Pichia pastoris. Version M
Kim T, Mullaney EJ, Porres JM, Roneker KR, Crowe S, Rice S, Ko T, Ullah AHJ, Daly CB, Welch R, Lei X (2006) Shifting the pH profile of Aspergillus niger PhyA phytase to match the stomach pH enhances its effectiveness as an animal feed additive. Appl Environ Microbiol 72:4397–4403
Lee DY, Schroeder J, Gordon DT (1988) Enhancement of Cu bioavailability in the rat by phytic acid. J Nutr 118:712–717
Lehmann M, Pasamontes L, Lassen SF, Wyss M (2000) The consensus concept for thermostability engineering of proteins. Biochim Biophys Acta 1543:408–415
Lei X, Stahl CH (2001) Biotechnological development of effective phytases for mineral nutrition and environmental protection. Appl Microbiol Biotechnol 57:474–481
Lei X, Ku PK, Miller ER, Ullrey DE, Yokoyama MT (1993a) Supplemental microbial phytase improves bioavailability of dietary zinc to weanling pigs. J Nutr 123:1117–1123
Lei X, Ku PK, Miller ER, Yokoyama MT, Ullrey DE (1993b) Supplementing corn-soybean meal diets with microbial phytase maximizes phytate phosphorus utilization by weanling pigs. J Anim Sci 71:3368–3375
Lim D, Golovan S, Forsberg CW, Jia ZC (2000) Crystal structures of Escherichia coli phytase and its complex with phytate. Nat Struct Biol 7:108–113
Liu YG, Whittier RF (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681
Maenz DD, Classen HL (1998) Phytase activity in the small intestinal brush border membrane of the chicken. Poult Sci 77:557–563
Minekus M, Marteau P, Havenaar R, Huis in’t Veld JHJ (1995) A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Altern Lab Ani 23:197–209
Mullaney EJ, Daly CB, Kim T, Porres JM, Lei X, Sethumadhavan K, Ullah AHJ (2002) Site-directed mutagenesis of Aspergillus niger NRRL 3135 phytase at residue 300 to enhance catalysis at pH 4.0. Biochem Biophys Res Commun 297:1016–1020
Nelson TS, Shieh TR, Wodzinski RJ, Ware JH (1971) Effect of supplemental phytase on the utilization of phytate phosphorus by chicks. J Nutr 101:1289–1293
Pagano AR, Roneker KR, Lei X (2007) Distribution of supplemental Escherichia coli AppA2 phytase activity in digesta of various gastrointestinal segments of young pigs. J Anim Sci 85:1444–1452
Pharmacopeia US (2000) Simulated gastric fluid, TS. National Formulary no. 24/19, 2235. National Publishing, Philadelphia, Pa
Reddy NR, Sathe SK, Salunkhe DK (1982) Phytates in legumes and cereals. Adv Food Res 28:1–92
Rodriguez E, Porres JM, Han YM, Lei X (1999) Different sensitivity of recombinant Aspergillus niger phytase (r-PhyA) and Escherichia coli pH 2.5 acid phosphatase (r-AppA) to trypsin and pepsin in vitro. Arch Biochem Biophys 365:262–267
Rodriguez E, Wood ZA, Karplus PA, Lei X (2000) Site-directed mutagenesis improves catalytic efficiency and thermostability of Escherichia coli pH 2.5 acid phosphatase/phytase expressed in Pichia pastoris. Arch Biochem Biophys 382:105–112
Simons PC, Versteegh HA, Jongbloed AW, Kemme PA, Slump P, Bos KD, Wolters MG, Beudeker RF, Verschoor GJ (1990) Improvement of phosphorus availability by microbial phytase in broilers and pigs. Br J Nutr 64:525–540
Tomschy A, Brugger R, Lehmann M, Svendsen A, Vogel K, Kostrewa D, Lessen SF, Burger D, Kronenberger A, van Loon APGM, Pasamontes L, Wyss M (2002) Engineering of phytase for improved activity at low pH. Appl Environ Microbiol 68:1907–1913
Wodzinski RJ, Ullah AHJ (1996) Phytase. Adv Appl Microbiol 42:263–302
Xiong AS, Yao QH, Peng RH, Zhang Z, Xu F, Liu JG, Han PL, Chen JM (2006) High level expression of a synthetic gene encoding Peniophora lycii phytase in methylotrophic yeast Pichia pastoris. Appl Microbiol Biotechnol 72:1039–1047
Yi Z, Kornegay ET (1996) Sites of phytase activity in the gastrointestinal tract of young pigs. Anim Feed Sci Tech 61:361–368
Zhang W, Mullaney EJ, Lei X (2007) Adopting selected hydrogen bonding and ionic interactions from Aspergillus fumigatus phytase structure improves thermostability of Aspergillus niger PhyA phytase. Appl Environ Microbiol 73:3069–3076
Acknowledgments
This research was supported by the National Basic Research Program of China (973 Program, Grant No. 2004CB719606) and the National High Technology Research and Development Program of China (863 program, Grant No. 2006AA020101). We thank Professor Yang RF and Wang XY of the Academy of Military Medical Sciences for providing genomic DNA of Yersinia spp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, H., Luo, H., Wang, Y. et al. A novel phytase from Yersinia rohdei with high phytate hydrolysis activity under low pH and strong pepsin conditions. Appl Microbiol Biotechnol 80, 417–426 (2008). https://doi.org/10.1007/s00253-008-1556-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1556-5