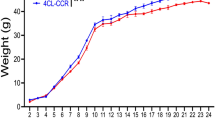
Abstract
Laccase (Lcc) is a lignin-degrading enzyme produced by white-rot fungi and has been the subject of much interest in the field of bioremediation due to its ability to oxidize phenolic compounds. In this report, we describe the isolation and characterization of lcc1, a novel gene of Lentinula edodes that encodes Lcc1, and demonstrate that recombinant Lcc1 is expressed in an active, secreted form in tobacco BY-2 cells in culture. The open reading frame of lcc1 was 1,557 base pairs in length and encoded a putative protein of 518 amino acids. We introduced a chimeric form of lcc1 (CaMV35Sp:clcc1) into tobacco BY-2 cells and obtained several stable clcc1 transformants that expressed active Lcc1. Lcc1 activity in BY-2 culture media was higher than in cellular extracts, which indicated that recombinant Lcc1 was produced in a secreted form. Recombinant Lcc1 had a smaller apparent molecular weight and exhibited a different pattern of posttranslational modification than Lcc1 purified from L. edodes. The substrate specificity of purified recombinant Lcc1 was similar to L. edodes Lcc1, and both enzymes were able to decolorize the same set of dyes. These results suggest that heterologous expression of fungal Lcc1 in BY-2 cells will be a valuable tool for the production of sufficient quantities of active laccase for bioremediation.







Similar content being viewed by others
References
Alves AM, Record E, Lomascolo A, Scholtmeijer K, Asther M, Wessels JG, Wösten HA (2004) Highly efficient production of laccase by the basidiomycete Pycnoporus cinnabarinus. Appl Environ Microbiol 70:6379–6384
Bailey MR, Wooddard SL, Callaway E, Beifuss K, Magallanes-Lundback M, Lane JR, Horn ME, Mallubhotla H, Delaney DD, Ward M, Van Gastel F, Howard JA, Hood EE (2004) Improved recovery of active recombinant laccase from maize seed. Appl Microbiol Biotechnol 63:390–397
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30:215–242
Bulter T, Alcalde M, Sieber V, Meinhold P, Schlachtbauer C, Aenold FH (2003) Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Appl Environ Microbiol 69:987–995
Camarero S, Ibarra D, Martinez MJ, Martinez AT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71:1775–1784
Christian V, Shribastava R, Shukla D, Modi HA, Vyas BRM (2005) Degradation of xenobiotic compounds by lignin-degrading white-rot fungi: enzymology and mechanisms involved. Indian J Exp Biol 43:301–312
Collins PJ, Dobson ADW (1997) Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol 63:3444–3450
Couto SR, Toca-Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Geng X, Li K, Xu F (2004) Investigation of hydroxamic acids as laccase-mediators for pulp bleaching. Appl Microbiol Biotechnol 64:493–496
Gurr SJ, Unkles SEU, Kinghorn JR (1987) The structure and organization of nuclear genes of filamentous fungi. In: Kinghorn JR (ed) Gene structure in eukaryotic microbes. IRL, London, pp 93–139
Hirano T, Sato T, Okawa K, Kanda K, Yaegashi K, Enei H (1999) Isolation and characterization of the glyceraldehyde-3-phosphate dehydrogenase gene of Lentinula edodes. Biosci Biotechnol Biochem 63:1223–1227
Isegawa Y, Sheng J, Sokawa Y, Yamanishi K, Nakagomi O, Shigeharu U (1992) Selective amplification of cDNA sequence from total RNA by cassette-ligation mediated polymerase chain reaction (PCR): application to sequencing 6.5 kb genome segment of hantavirus strain B-1. Mol Cell Probes 6:467–475
Jolivalt C, Madzak C, Brault A, Caminade E, Malosse C, Mougin C (2005) Expression of laccase IIIb from the white-rot fungus Trametes versicolor in the yeast Yarrowia lipolytica for environmental applications. Appl Microbiol Biotechnol 66:450–456
Jonsson L, Sjostrom K, Haggstrom I, Nyman PO (1995) Characterization of a laccase gene from the white-rot fungus Trametes versicolor and structural features of basidiomycete laccases. Biochim Biophys Acta 1251:210–215
Krcˇmrˇ P, Ulrich R (1998) Degradation of polychlorinated biphenyl mixtures by the lignin-degrading fungus Phanerochaete chrysosporium. Folia Microbiol 43:79–84
Kumar SVS, Phale PS, Durani S, Wangikar P (2003) Combined sequence and structure analysis of the fungal laccase family. Bioetchnol Bioengin 83:386–394
Leonowicz A, Cho NS, Luterek J, Wilkolazka A, Wojtas-Wasilewska M, Matuszewska A, Hofrichter M, Wesenberg D, Rogalski J (2001) Fungal laccase: properties and activity on lignin. J Basic Microbiol 41:185–227
Marillonnet S, Giritch A, Gils M, Kandzia R, Klimyuk V, Gleba Y (2004) In planta engineering of viral RNA replicons: efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc Natl Acad Sci U S A 101:6852–6857
Marillonnet S, Thoeringer C, Kandzia R, Klimyuk V, Gleba Y (2005) Viral-mediated transformation gets a boost. Nat Biotechnol 23:718–723
Ministry of the Environments (2005) http://www.env.go.jp/air/tech/bio/ (in Japanese)
Ministry of the Environments (2007) Regulations related to the enforcement of the law concerning the conservation and sustainable use of biological diversity through regulations on the use of living modified organisms (Tentative Translation) http://www.env.go.jp/en/laws/nature/reg_ccsubdrlmo.pdf
Ministry of the Environments, Environmental Management Bureau (2006) The results of the survey on enforcement status of the soil contamination countermeasures act and numbers and trends of soil contamination investigations and countermeasures in the fiscal year 2004. http://www.env.go.jp/en/water/soil/result/2004_full.pdf
Morisaki K, Fushimi T, Kaneko S, Kusakabe I, Kobayashi H (2001) Screening for phenoloxidases from edible mushrooms. Biosci Biotechnol Biochem 65:2334–2336
Nagai M, Sato T, Watanabe H, Saito K, Kawata M, Enei H (2002) Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl Microbiol Biotechnol 60:327–335
Nagai M, Kawata M, Watanabe H, Ogawa M, Saito K, Takesawa T, Kanda K, Sato T (2003) Important role of fungal intracellular laccase for melanin synthesis: purification and characterization of an intracellular laccase from Lentinula edodes fruit bodies. Microbiology 149:2455–2462
Nagai M, Sato T, Enei H (2004) Bioremediation by laccases Lentinula edodes. Mushroom Sci 16:573–578
Palmieri G, Giardina P, Bianco C, Fontanella B, Sannia G (2000) Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl Environ Microbiol 66:920–924
Sack M, Paetz A, Kunert R, Bomble M, Hesse F, Stiegler G, Fischer R, Katinger H, Stoeger E, Rademacher T (2007) Functional analysis of the broadly neutralizing human anti-HIV-1 antibody 2F5 produced in transgenic BY-2 suspension cultures. FASEB J 21:1655–1664
Sakamoto Y, Irie T, Sato T (2005a) Isolation and characterization of a fruiting body specific exo-b-1, 3-glucanase-encoding gene, exg1, from Lentinula edodes. Curr Genet 47:244–252
Sakamoto Y, Minato K, Nagai M, Mizuno M, Sato T (2005b) Characterization of the Lentinula edodes exg2 gene encoding a lentinan-degrading exo-b-1, 3-glucanase. Curr Genet 48:195–203
Sato Y, Whetten RW (2006) Characterization of two laccases of loblolly pine (Pinus taeda) expressed in tobacco BY-2 cells. J Plant Res 119:581–588
Sigoillot C, Record E, Belle V, Robert JL, Levasseur A, Punt PJ, van den Hondel CAMJJ, Fournel A, Sigoillot JC, Asther M (2004) Natural and recombinant fungal laccases for paper pulp bleaching. Appl Microbiol Biotechnol 64:346–352
Soden DM, Dobson ADW (2001) Differential regulation of laccase gene expression in Pleurotus sajor-caju. Microbiol 147:1755–1763
Soden DM, O, Callaghan J, Dobson DW (2002) Molecular cloning of a laccase isozyme gene from Pleurotus sajor-caju and expression in the heterologous Pichia pastoris host. Microbiol 148:4003–4014
Sonoki T, Kajita S, Ikeda S, Uesugi M, Tatsumi K, Katayama Y, Iimura Y (2005) Transgenic tobacco expressing fungal laccase promotes the detoxification of environmental pollutants. Appl Microbiol Biotechnol 67:138–142
Takada S, Nakamura M, Matsueda T, Kondo R, Sakai K (1996) Degradation of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans by the white rot fungus Phanerochaete sordida YK-624. Appl Environ Micobiol 62:4323–4328
Ullah MA, Bedford CT, Evans CS (2000) Reactions of pentachlorophenol with laccase from Coriolus versicolor. Appl Microbiol Biotechnol 53:230–234
Vaquero C, Sack M, Chandler J, Drossard J, Schuster F, Monecle M, Schillberg S, Fischer R (1999) Transient expression of a tumor-specific single-chain fragment and a chimeric antibody in tobacco leaves. Proc Natl Acad Sci 96:11128–11133
Wang GD, Li QJ, Luo B, Chen XY (2004) Ex planta phytoremediation of trichlorophenol and phenolic allelochemicals via an engineered secretory laccase. Nat Biotech 22:893–897
Zhao J, Kwan HS (1999) Characterization, molecular cloning, and differential expression analysis of laccase genes from Lentinula edodes. Appl Environ Microbiol 65:4908–4913
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1.
Nucleotide sequence and deduced amino acid sequence of L. edodes lccl gene. Red letters: signal peptide, blue letters: cupper binding site, green letters: N-glycosylation site (JPEG 1.41 MB)
Supplementary Figure 2.
Southern blot analysis of L. edodes lccl gene. Full length of lccl cDNA was used for the probe. In the probe, there are no recognition sequences of all restriction enzymes used for southern bolt analysis. (JPEG 308 KB)
Supplementary Figure 3.
Northern blot analysis of L. edodes lccl gene. Northern blot analysis was carried out by the method of Hirano et al. (1999). 1; vegetative mycelia, 2; primordia, 3; stipe of mature fruit body, 4; gill of mature fruit body, 5; pileus of mature fruit body. gpd: glyceraldehyde-3-phosphate dehydrogenase gene. ras: ras gene (JPEG 503 KB)
Rights and permissions
About this article
Cite this article
Sakamoto, Y., Nakade, K., Yano, A. et al. Heterologous expression of lcc1 from Lentinula edodes in tobacco BY-2 cells results in the production an active, secreted form of fungal laccase. Appl Microbiol Biotechnol 79, 971–980 (2008). https://doi.org/10.1007/s00253-008-1507-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1507-1