Abstract
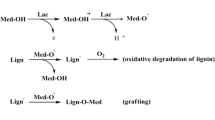
Three laccases, a natural form and two recombinant forms obtained from two different expression hosts, were characterized and compared for paper pulp bleaching. Laccase from Pycnoporus cinnabarinus, a well known lignolytic fungus, was selected as a reference for this study. The corresponding recombinant laccases were produced in Aspergillus oryzae and A. niger hosts using the lacI gene from P. cinnabarinus to develop a production process without using the expensive laccase inducers required by the native source. In flasks, production of recombinant enzymes by Aspergilli strains gave yields close to 80 mg l−1. Each protein was purified to homogeneity and characterized, demonstrating that the three hosts produced proteins with similar physico-chemical properties, including electron paramagnetic resonance spectra and N-terminal sequences. However, the recombinant laccases have higher Michaelian (K m) constants, suggesting a decrease in substrate/enzyme affinity in comparison with the natural enzyme. Moreover, the natural laccase exhibited a higher redox potential (around 810 mV), compared with A. niger (760 mV) and A. oryzae (735 mV). Treatment of wheat straw Kraft pulp using laccases expressed in P. cinnabarinus or A. niger with 1-hydroxybenzotriazole as redox mediator achieved a delignification close to 75%, whereas the recombinant laccase from A. oryzae was not able to delignify pulp. These results were confirmed by thioacidolysis. Kinetic and redox potential data and pulp bleaching results were consistent, suggesting that the three enzymes are different and each fungal strain introduces differences during protein processing (folding and/or glycosylation).




Similar content being viewed by others
References
Berka RM, Schneider P, Golightly EJ, Brown SH, Madden M, Brown KM, Halkier T, Mondorf K, Xu F (1997) Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl Environ Microbiol 63:3151–3157
Bonnarme P, Moukha S, Moreau P, Record E, Lesage L, Cassagne C, Asther M (1994) Fractionation of subcellular membranes of the secretory pathway from the peroxidase-producing white rot fungus Phanerochaete chrysosporium. FEMS Microbiol Lett 120:155–162
Bourbonnais R, Leech D, Paice MG (1998) Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim Biophys Acta 1379:381–390
Call HP, Mücke I (1997) History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (Lygnozym-process). J Biotechnol 53:163–202
Ducros V, Davies JG, Lawson DM, Brown SH, Østergaard P, Pedersen AH, Schneider P, Yaver DS, Marek Brzozowski A (1987) Crystallisation and preliminary X-ray analysis of the laccase from Coprinus cinereus. Acta Crystallogr D 53:605–607
Ducros V, Brzozowski AM, Wilson KS, Brown SH, Østergaard P, Schneider P, Yaver AH, Pederson AH, Davies GJ (1998) Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat Struct Biol 5:310–316
Eggert C, Temp U, Eriksson KE (1996) The lignolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Fabbrini M, Galli C, Gentili P (2002) Comparing the catalytic efficiency of some mediators of laccase. J Mol Catal B Enzym 16:231–240
Gianfreda L, Xu F, Bollag JM (1999) Laccases: a useful group of oxidoreductive enzymes. Bioremediation J 3:1–25
Herpoël I, Moukha S, Lesage-Meesen L, Sigoillot JC, Asther M (2000) Selection of Pycnoporus cinnabarinus strains for laccase production. FEMS Microbiol Lett 183:301–306
Herpoël I, Jeller H, Fang G, Petit-Conil M, Bourbonnais R, Robert JL, Asther M, Sigoillot JC (2002) Efficient enzymatic delignification of wheat straw pulp by a sequential xylanase-laccase mediator treatment. J Pulp Pap Sci 28:67–71
Johannes C, Majcherczyk A (2000) Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl Environ Microbiol 66:524–528
Kanbi LD, Antonyuk S, Hough MA, Hall JF, Dodd FE, Hasnain SS (2002) Crystal structures of the Met148Leu and Ser86Asp mutants of rusticyanin from Thiobacillus ferrooxidans: insights into the structural relationship with the cupredoxins and the multi copper proteins. J Mol Biol 320:263–275
Laemmli, UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lapierre C, Monties B, Rolando C (1986) Thioacidolysis of poplar lignins: identification of monomeric syringyl products and characterization of guaiacyl–syringyl lignin fractions. Holzforschung 40:113–118
Li K, Xu F, Eriksson KEL (1999) Comparison of fungal laccases and redox mediators in oxidation of a non phenolic lignin model compound. Appl Environ Microbiol 65:2654–2660
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Otterbein L, Record E, Chereau D, Herpoël I, Asther M, Moukha S (2000) Isolation of a new laccase isoform from the white-rot fungi Pycnoporus cinnabarinus strain ss3. Can J Microbiol 46:759–763
Piontek K, Antorini M, Choinowski T (2002) Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J Biol Chem 40:37663–37669
Punt PJ, Hondel CAAJJ van den (1992) Transformation of filamentous fungi based on hygromycin B and phleomycin resistance markers. Methods Enzymol 216:447–457
Record E, Punt PJ, Chamkha M, Labat M, Hondel CAAJJ van den, Asther M (2002) Expression of the Pycnoporus cinnabarinus laccase gene in Aspergillus niger and characterization of the recombinant enzyme. Eur J Biochem 269:602–609
Reinhammar B (1984) Laccase. In :Lontie R (ed) Copper proteins and copper enzymes, vol 3. CRC Press, Boca Raton, pp 1–35
Sigoillot JC, Herpoël I, Frasse P, Moukha S, Lesage-Messen L, Asther M (1999) Laccase production by a monokaryotic strain of Pycnoporus cinnabarinus derived from a dikaryotic strain. World J Microbiol Biotechnol 15:481–484
Solomon EI, Chen P, Metz M, Lee SK, Palmer AE (2001) Oxygen binding, activation, and reduction to water by copper proteins Angew Chem Int Ed Engl 40:4570–4590
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 140:19–25
Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI (1996) A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta 1292:303–311
Yaver DS, Xu F, Golightly EJ, Brown SH, Rey MW, Schneider P, Halkier T, Mondorf K, Dalboge H (1996) Purification, characterization, molecular cloning and expression of two laccases from the white rot basidiomycete Trametes villosa. Appl Environ Microbiol 62:834–841
Yoshida H (1883) Chemistry of lacquer (urichi). J Chem Soc 43:472–486
Acknowledgements
We thank P. Mansuelle (UMR 6560, IFR Jean Roche, Marseille, France) for the N-terminal sequence analysis. This research was supported by the EU Quality of Living Resources programme (Project QLK3-99-590, Fungal metalloenzymes oxidizing aromatic compound of industrial interest) and GIS-EBL (Région Provence Alpes Côte d’Azur and Conseil Général des Bouches-du-Rhône, France). C.S. is grateful to the Conseil Régional Provence-Alpes-Côte d’Azur, France, the Institut National de la Recherche Agronomique and Tembec-Tarascon S.A. for a PhD scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sigoillot, C., Record, E., Belle, V. et al. Natural and recombinant fungal laccases for paper pulp bleaching. Appl Microbiol Biotechnol 64, 346–352 (2004). https://doi.org/10.1007/s00253-003-1468-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1468-3