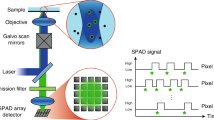
Abstract
The spatial and temporal fluctuation microscope (STFM) presented here extends the concept of a fluorescence confocal laser scanning microscope to illumination and detection along a line. The parallel multichannel acquisition of the fluorescence signal was accomplished by using a single line of an electron-multiplying charge-coupled device camera at 14 μs time resolution for detection of the fluorescence signal. The STFM system provided fast confocal imaging (30 images per second) and allowed for the spatially resolved detection of particle concentration fluctuations in fluorescence correlation spectroscopy experiments. For the application of the STFM, an approximated theoretical description of the beam geometry, the point-spread function, and the fluorescence auto- and cross-correlation functions were derived. The STFM was applied to studies of the dynamics of promyelocytic leukemia nuclear bodies, green fluorescent protein, and chromatin-remodeling complexes in living cells. The results demonstrate the unique capabilities of the STFM for characterizing the position-dependent translocations and interactions of proteins in the cell.






Similar content being viewed by others
Abbreviations
- GFP:
-
Green fluorescent protein
- MSD:
-
Mean-squared displacement
- SPT:
-
Single-particle tracking
- FRAP:
-
Fluorescence recovery after photobleaching
- FCS:
-
Fluorescence correlation spectroscopy
- FCCS:
-
Fluorescence cross-correlation spectroscopy
- STFM:
-
Spatial and temporal fluctuation microscope/microscopy
- CLSM:
-
Fluorescence confocal laser-scanning microscope
- EM-CCD:
-
Electron-multiplying charge-coupled device
- PML-NB:
-
Promyelocytic leukemia nuclear body
- PSF:
-
Point-spread function
- TIRF:
-
Total internal reflection fluorescence
References
Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW (1976) Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J 16:1055–1069
Bayer J, Radler JO (2006) DNA microelectrophoresis using double focus fluorescence correlation spectroscopy. Electrophoresis 27:3952–3963
Beaudouin J, Mora-Bermúdez F, Klee T, Daigle N, Ellenberg J (2006) Dissecting the contribution of diffusion and interactions to the mobility of nuclear proteins. Biophys J 90:1878–1894
Becker PB, Horz W (2002) ATP-dependent nucleosome remodeling. Annu Rev Biochem 71:247–273
Berland KM, So PT, Gratton E (1995) Two-photon fluorescence correlation spectroscopy: method and application to the intracellular environment. Biophys J 68:694–701
Berland KM, So PTC, Chen Y, Mantulin WW, Gratton E (1996) Scanning 2-photon fluctuation correlation spectroscopy—particle counting measurements for detection of molecular aggregation. Biophys J 71:410–420
Borlinghaus RT (2006) High speed scanning has the potential to increase fluorescence yield and to reduce photobleaching. Micr Res Tech 69:689–692
Brinkmeier M, Dorre K, Riebeseel K, Rigler R (1997) Confocal spectroscopy in microstructures. Biophys Chem 66:229–239
Brinkmeier M, Dorre K, Stephan J, Eigen M (1999) Two beam cross correlation: a method to characterize transport phenomena in micrometer-sized structures. Anal Chem 71:609–616
Brown CM, Dalal RB, Hebert B, Digman MA, Horwitz AR, Gratton E (2008) Raster image correlation spectroscopy (RICS) for measuring fast protein dynamics and concentrations with a commercial laser scanning confocal microscope. J Microsc 229:78–91
Burkhardt M, Schwille P (2006) Electron multiplying CCD based detection for spatially resolved fluorescence correlation spectroscopy. Opt Exp 14:5013–5020
Cairns BR (2007) Chromatin remodeling: insights and intrigue from single-molecule studies. Nat Struct Mol Biol 14:989–996
Cole NB, Smith CL, Sciaky N, Terasaki M, Edidin M, Lippincott-Schwartz J (1996) Diffusional mobility of Golgi proteins in membranes of living cells. Science 273:797–801
Collins N, Poot RA, Kukimoto I, Garcia-Jimenez C, Dellaire G, Varga-Weisz PD (2002) An ACF1-ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nat Genet 32:627–632
Cutts LS, Roberts PA, Adler J, Davies MC, Melia CD (1995) Determination of localized diffusion coefficients in gels using confocal scanning laser microscopy. J Microsc 180:131–139
Digman MA, Brown CM, Sengupta P, Wiseman PW, Horwitz AR, Gratton E (2005a) Measuring fast dynamics in solutions and cells with a laser scanning microscope. Biophys J 89:1317–1327
Digman MA, Sengupta P, Wiseman PW, Brown CM, Horwitz AR, Gratton E (2005b) Fluctuation correlation spectroscopy with a laser-scanning microscope: exploiting the hidden time structure. Biophys J 88:L33–L36
Dusch E, Dorval T, Vincent N, Wachsmuth M, Genovesio A (2007) Three-dimensional point spread function model for line-scanning confocal microscope with high-aperture objective. J Microsc 228:132–138
Elson EL, Magde D (1974) Fluorescence correlation spectroscopy. I: Conceptual basis and theory. Biopolymers 13:1–27
Gomez DE, Califano M, Mulvaney P (2006) Optical properties of single semiconductor nanocrystals. Phys Chem Chem Phys 8:4989–5011
Görisch SM, Wachsmuth M, Ittrich C, Bacher CP, Rippe K, Lichter P (2004) Nuclear body movement is determined by chromatin accessibility and dynamics. Proc Natl Acad Sci USA 101:13221–13226
Görisch SM, Lichter P, Rippe K (2005) Mobility of multi-subunit complexes in the nucleus: chromatin dynamics and accessibility of nuclear subcompartments. Histochem Cell Biol 123:217–228
Gunkel M, Erdel F, Rippe K, Lemmer P, Kaufmann R, Hörmann C, Amberger R, Cremer C (2009) Dual color localization microscopy of cellular nanostructures. Biotechnol J (in press)
Hecht E (1989) Optics. Addisson-Wesley, Longmann, New York
Hess ST, Webb WW (2002) Focal volume optics and experimental artifacts in confocal fluorescence correlation spectroscopy. Biophys J 83:2300–2317
Heuff RF, Swift JL, Cramb DT (2007) Fluorescence correlation spectroscopy using quantum dots: advances, challenges and opportunities. Phys Chem Chem Phys 9:1870–1880
Heuvelman G (2008) Development and design of a spatially and temporally resolved fluorescence fluctuation microscope for the analysis of molecular mobilities and interactions. PhD Thesis, Ruprecht-Karls-Universität Heidelberg, Heidelberg
Hwang LC, Wohland T (2007) Recent advances in fluorescence cross-correlation spectroscopy. Cell Biochem Biophys 49:1–13
Jegou T, Chung I, Heuvelmann G, Wachsmuth M, Görisch SM, Greulich-Bode K, Boukamp P, Lichter P, Rippe K (2009) Dynamics of telomeres and promyelocytic leukemia nuclear bodies in a telomerase negative human cell line. Mol Biol Cell 20:2070–2082
Kannan B, Guo L, Sudhaharan T, Ahmed S, Maruyama I, Wohland T (2007) Spatially resolved total internal reflection fluorescence correlation microscopy using an electron multiplying charge-coupled device camera. Anal Chem 79:4463–4470
Kolin DL, Wiseman PW (2007) Advances in image correlation spectroscopy: measuring number densities, aggregation states, and dynamics of fluorescently labeled macromolecules in cells. Cell Biochem Biophys 49:141–164
Kudryavtsev V, Felekyan S, Wozniak AK, Konig M, Sandhagen C, Kuhnemuth R, Seidel CA, Oesterhelt F (2007) Monitoring dynamic systems with multiparameter fluorescence imaging. Anal Bioanal Chem 387:71–82
Lamond AI, Sleeman JE (2003) Nuclear substructure and dynamics. Curr Biol 13:R825–R828
Längst G, Becker PB (2001) Nucleosome mobilization and positioning by ISWI-containing chromatin-remodeling factors. J Cell Sci 114:2561–2568
LeCaptain DJ, Van Orden A (2002) Two-beam fluorescence cross-correlation spectroscopy in an electrophoretic mobility shift assay. Anal Chem 74:1171–1176
Lumma D, Best A, Gansen A, Feuillebois F, Radler JO, Vinogradova OI (2003) Flow profile near a wall measured by double-focus fluorescence cross-correlation. Phys Rev E Stat Nonlin Soft Matter Phys 67:056313
Magde D, Elson EL, Webb WW (1972) Thermodynamic fluctuations in a reacting system—measurement by fluorescence correlation spectroscopy. Phys Rev Lett 29:705–708
Magde D, Elson EL, Webb WW (1974) Fluorescence correlation spectroscopy. II: An experimental realization. Biopolymers 13:29–61
Pack C, Saito K, Tamura M, Kinjo M (2006) Microenvironment and effect of energy depletion in the nucleus analyzed by mobility of multiple oligomeric EGFPs. Biophys J 91:3921–3936
Palmer AG, Thompson NL (1987) Theory of sample translation in fluorescence correlation spectroscopy. Biophys J 51:339–343
Pan X, Foo W, Lim W, Fok MH, Liu P, Yu H, Maruyama I, Wohland T (2007) Multifunctional fluorescence correlation microscope for intracellular and microfluidic measurements. Rev Sci Instrum 78:053711
Pawley JB (ed) (1995) Handbook of biological confocal microscopy, 2nd ed. Plenum, New York
Peters R, Peters J, Tews KH, Bahr W (1974) A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta 367:282–294
Peters R, Brünger A, Schulten K (1981) Continuous fluorescence microphotolysis: a sensitive method for study of diffusion processes in single cells. Proc Natl Acad Sci USA 78:962–966
Petrasek Z, Schwille P (2008) Precise measurement of diffusion coefficients using scanning fluorescence correlation spectroscopy. Biophys J 94:1437–1448
Qian H, Elson EL (1991) Analysis of confocal laser-microscope optics for 3-D fluorescence correlation spectroscopy. Appl Opt 30:1185–1195
Rippe K, Schrader A, Riede P, Strohner R, Lehmann E, Langst G (2007) DNA sequence- and conformation-directed positioning of nucleosomes by chromatin-remodeling complexes. Proc Natl Acad Sci USA 104:15635–15640
Ruan Q, Cheng MA, Levi M, Gratton E, Mantulin WW (2004) Spatial-temporal studies of membrane dynamics: scanning fluorescence correlation spectroscopy (SFCS). Biophys J 87:1260–1267
Schwille P (2003) TIR-FCS: staying on the surface can sometimes be better. Biophys J 85:2783–2784
Sisan DR, Arevalo R, Graves C, McAllister R, Urbach JS (2006) Spatially resolved fluorescence correlation spectroscopy using a spinning disk confocal microscope. Biophys J 91:4241–4252
Skinner JP, Chen Y, Muller JD (2005) Position-sensitive scanning fluorescence correlation spectroscopy. Biophys J 89:1288–1301
van Holde KE (1989) Chromatin. Springer, Heidelberg
Wachsmuth M, Weisshart K (2007) Fluorescence photobleaching and fluorescence correlation spectroscopy: two complementary technologies to study molecular dynamics in living cells. In: Shorte SL, Frischknecht F (eds) Imaging cellular and molecular biological functions. Springer, Heidelberg
Wachsmuth M, Waldeck W, Langowski J (2000) Anomalous diffusion of fluorescent probes inside living cell nuclei investigated by spatially-resolved fluorescence correlation spectroscopy. J Mol Biol 298:677–689
Wachsmuth M, Weidemann T, Muller G, Hoffmann-Rohrer UW, Knoch TA, Waldeck W, Langowski J (2003) Analyzing intracellular binding and diffusion with continuous fluorescence photobleaching. Biophys J 84:3353–3363
Wachsmuth M, Caudron-Herger M, Rippe K (2008) Genome organization: balancing stability and plasticity. Biochim Biophys Acta 1783:2061–2079
Acknowledgments
We are indebted to Christoph Cremer for his continuous support of the development of the STFM instrument at the Kirchhoff-Institut für Physik, and thank Felix Bestvater, Zahir Seghiri, Moon Sik Kang, Katharina Müller, and Michael Tewes for help and discussions as well as Werner Knebel from Leica Microsystems and Peter Vogt from Coherent for technical support. The detailed comments and suggestions of one of the reviewers for the revision of the paper are gratefully acknowledged. The work was funded by the Volkswagenstiftung and by the Deutsche Forschungsgemeinschaft within the Research Training Group “Molecular imaging methods for the analysis of gene and protein expression” (GRK 886/1) and by grants Ri 1283/5-3 (SPP1128 priority program “Optical analysis of structure and dynamics of supramolecular biological complexes”) and Ri 1283/8-1.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article has been submitted as a contribution to the festschrift entitled “Uncovering cellular sub-structures by light microscopy” in honour of Professor Cremer’s 65th birthday.
Rights and permissions
About this article
Cite this article
Heuvelman, G., Erdel, F., Wachsmuth, M. et al. Analysis of protein mobilities and interactions in living cells by multifocal fluorescence fluctuation microscopy. Eur Biophys J 38, 813–828 (2009). https://doi.org/10.1007/s00249-009-0499-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-009-0499-9