Abstract
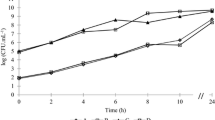
Listeria monocytogenes is a foodborne pathogen that can be transmitted through contaminated raw food or by ready-to-eat products that have been in contact with contaminated surfaces. Tap water (TW) is used to wash produce, as a processed food constituent and to wash processing surfaces and floors. The main aim of this work was to investigate the formation and survival of L. monocytogenes biofilms on stainless steel (SS) coupons in TW at 4, 22, 30 and 37 °C. For that, coupons with biofilm were visualised in situ while other coupons were scraped to quantify total cells by SYTO 9, cultivable numbers by plating onto brain heart infusion agar and viable numbers by the direct viable count method. Results showed that L. monocytogenes can form biofilms on SS surfaces in TW at any temperature, including at 4 °C. The number of total cells was similar for all the conditions tested while cultivable numbers varied between the level of detection (<8.3 CFU cm−2) and 3.5 × 105 CFU cm−2, meaning between 7.0 × 104 and 1.1 × 107 cells cm−2 have entered the viable but non-cultivable (VBNC) state. This work clearly demonstrates that L. monocytogenes can form biofilms in TW and that sessile cells can remain viable and cultivable in some conditions for at least the 48 h investigated. On the other hand, VBNC adaptation suggests that the pathogen can remain undetectable using traditional culture recovery techniques, which may give a false indication of processing surface hygiene status, leading to potential cross-contamination of food products.




Similar content being viewed by others
References
Gandhi M, Chikindas ML (2007) Listeria: a foodborne pathogen that knows how to survive. Int J Food Microbiol 113:1–15
Farber JM, Peterkin PI (1991) Listeria monocytogenes, a food-borne pathogen. Microbiol Rev 55:476–511
Nørrung B (2000) Microbiological criteria for Listeria monocytogenes in foods under special consideration of risk assessment approaches. Int J Food Microbiol 62:217–221
European Commission (2007) Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. Off J Eur Union L L322:12–29
European Commission (2005) Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off J Eur Union L L338:1–26
Dalton CB, Austin CC, Sobel J, Hayes PS, Bibb WF, Graves LM, Swaminathan B, Proctor ME, Griffin PM (1997) An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med 336:100–106
Harvey J, Gilmour A (1994) Application of multilocus enzyme electrophoresis and restriction fragment length polymorphism analysis to the typing of Listeria monocytogenes strains isolated from raw milk, nondairy foods, and clinical and veterinary sources. Appl Environ Microbiol 60:1547–1553
Vitas AI, Aguado V, Garcia-Jalon I (2004) Occurrence of Listeria monocytogenes in fresh and processed foods in Navarra (Spain). Int J Food Microbiol 90:349–356
Watkins J, Sleath KP (1981) Isolation and enumeration of Listeria monocytogenes from sewage, sewage sludge and river water. J Appl Microbiol 50:1–9
Beresford MR, Andrew PW, Shama G (2001) Listeria monocytogenes adheres to many materials found in food-processing environments. J Appl Microbiol 90:1000–1005
Cox LJ, Kleiss T, Cordier JL, Cordellana C, Konkel P, Pedrazzini C, Beumer R, Siebenga A (1989) Listeria spp. in food processing, non-food and domestic environments. Food Microbiol 6:49–61
Beumer RR, te Giffel MC, Spoorenberg E, Rombouts FM (1996) Listeria species in domestic environments. Epidemiol Infect 117:437–442
Azevedo I, Regalo M, Mena C, Almeida G, Ls C, Teixeira P, Hogg T, Gibbs PA (2005) Incidence of Listeria spp. in domestic refrigerators in Portugal. Food Control 16:121–124
Borucki MK, Peppin JD, White D, Loge F, Call DR (2003) Variation in biofilm formation among strains of Listeria monocytogenes. Appl Environ Microbiol 69:7336–7342
Chae MS, Schraft H (2000) Comparative evaluation of adhesion and biofilm formation of different Listeria monocytogenes strains. Int J Food Microbiol 62:103–111
Stepanovic S, Cirkovic I, Ranin L, Svabic-Vlahovic M (2004) Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett Appl Microbiol 38:428–432
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890
Flemming H-C, Neu TR, Wozniak DJ (2007) The EPS matrix: the “house of biofilm cells”. J Bacteriol 189:7945–7947
Holah JT, Higgs C, Robinson S, Worthington D, Spenceley H (1990) A conductance-based surface disinfection test for food hygiene. Lett Appl Microbiol 11:255–259
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49:711–745
de Beer D, Srinivasan R, Stewart PS (1994) Direct measurement of chlorine penetration into biofilms during disinfection. Appl Environ Microbiol 60:4339–4344
Djordjevic D, Wiedmann M, McLandsborough LA (2002) Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol 68:2950–2958
Folsom JP, Siragusa GR, Frank JF (2006) Formation of biofilm at different nutrient levels by various genotypes of Listeria monocytogenes. J Food Prot 69:826–834
Kim KY, Frank JF (1995) Effect of nutrients on biofilm formation by Listeria monocytogenes on stainless steel. J Food Prot 58:24–28
Blackman IC, Frank JF (1996) Growth of Listeria monocytogenes as a biofilm on various food-processing surfaces. J Food Prot 59:827–831
Moltz AG, Martin SE (2005) Formation of biofilms by Listeria monocytogenes under various growth conditions. J Food Prot 68:92–97
Di Bonaventura G, Piccolomini R, Paludi D, D’Orio V, Vergara A, Conter M, Ianieri A (2008) Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: relationship with motility and cell surface hydrophobicity. J Appl Microbiol 104:1552–1561
Gião MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW (2008) Persistence of Helicobacter pylori in heterotrophic drinking water biofilms. Appl Environ Microbiol 74:5898–5904
Gião M, Wilks S, Azevedo N, Vieira M, Keevil C (2009) Validation of SYTO 9/Propidium Iodide Uptake for Rapid Detection of Viable but Noncultivable Legionella pneumophila. Microb Ecol 58:56–62
Oliver JD (2005) The viable but nonculturable state in bacteria. J Microbiol 43:93–100
Keevil CW (2003) Rapid detection of biofilms and adherent pathogens using scanning confocal laser microscopy and episcopic differential interference contrast microscopy. Water Sci Technol 47:105–116
Besnard V, Federighi M, Cappelier JM (2000) Development of a direct viable count procedure for the investigation of VBNC state in Listeria monocytogenes. Lett Appl Microbiol 31:77–81
Besnard V, Federighi M, Cappelier JM (2000) Evidence of viable but non-culturable state in Listeria monocytogenes by direct viable count and CTC-DAPI double staining. Food Microbiol 17:697–704
Mai TL, Conner DE (2007) Effect of temperature and growth media on the attachment of Listeria monocytogenes to stainless steel. Int J Food Microbiol 120:282–286
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209
Boulos L, Prevost M, Barbeau B, Coallier J, Desjardins R (1999) LIVE/DEAD(R) BacLight(™): application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J Microbiol Methods 37:77–86
Harmsen M, Lappann M, Knochel S, Molin S (2010) Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Appl Environ Microbiol 76:2271–2279
Vilain S, Brozel VS (2006) Multivariate approach to comparing whole-cell proteomes of Bacillus cereus indicates a biofilm-specific proteome. J Proteome Res 5:1924–1930
Varma PRG, Lyer TSG (1993) Viability of Listeria monocytogenes in water. Fish Technol 30:164–165
Botzler RG, Cowan AB, Wetzler TF (1974) Survival of Listeria monocytogenes in soil and water. J Wildl Dis 10:204–212
Azevedo NF, Almeida C, Fernandes I, Cerqueira L, Dias S, Keevil CW, Vieira MJ (2008) Survival of gastric and enterohepatic Helicobacter spp. in water: Implications for transmission. Appl Environ Microbiol 74:1805–1811
Buswell CM, Herlihy YM, Lawrence LM, McGuiggan JT, Marsh PD, Keevil CW, Leach SA (1998) Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl Environ Microbiol 64:733–741
Chae MS, Schraft H (2001) Cell viability of Listeria monocytogenes biofilms. Food Microbiol 18:103–112
Cappelier JM, Besnard V, Roche S, Garrec N, Zundel E, Velge P, Federighi M (2005) Avirulence of viable but non-culturable Listeria monocytogenes cells demonstrated by in vitro and in vivo models. Vet Res 36:589–599
Lindbäck T, Rottenberg ME, Roche SM, Rørvik LM (2010) The ability to enter into an avirulent viable but non-culturable (VBNC) form is widespread among Listeria monocytogenes isolates from salmon, patients and environment. Vet Res 41(1):8
Cappelier JM, Besnard V, Roche SM, Velge P, Federighi M (2007) Avirulent viable but non-culturable cells of Listeria monocytogenes need the presence of an embryo to be recovered in egg yolk and regain virulence after recovery. Vet Res 38:573–583
Lindbäck T, Secic I, Rørvik LM (2011) A contingency locus in prfA in a Listeria monocytogenes subgroup allows reactivation of the prfA virulence regulator during infection in mice. Appl Environ Microbiol 77:3478–3483
Chavant P, Martinie B, Meylheuc T, Bellon-Fontaine M-N, Hebraud M (2002) Listeria monocytogenes LO28: surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl Environ Microbiol 68:728–737
Sinde E, Carballo J (2000) Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: the influence of free energy and the effect of commercial sanitizers. Food Microbiol 17:439–447
Acknowledgments
The authors would like to thank Ms. Sonia Porta and Dr. Jose Belenguer from ainia—Centro Tecnologico (Spain)—and Mrs. Nuria de la Fuente and Dr. Enrique Orihuel from Betelgeux, S. L. (Spain) for the technical advice. This work was supported by the European Commission within the Seventh Framework Programme, ‘BioliSME—Speedy system for sampling and detecting Listeria monocytogenes in agri-food and related European industries’, no. FP7-SME-232037. The author is solely responsible for the work. It does not represent the opinion of the community, and the community is not responsible for any use that might be made of data appearing therein.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gião, M.S., Keevil, C.W. Listeria monocytogenes Can Form Biofilms in Tap Water and Enter Into the Viable but Non-Cultivable State. Microb Ecol 67, 603–611 (2014). https://doi.org/10.1007/s00248-013-0364-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-013-0364-3