Abstract
The ancient association of figs (Ficus spp.) and their pollinating wasps (fig wasps; Chalcidoidea, Hymenoptera) is one of the most interdependent plant–insect mutualisms known. In addition to pollinating wasps, a diverse community of organisms develops within the microcosm of the fig inflorescence and fruit. To better understand the multipartite context of the fig–fig wasp association, we used a culture-free approach to examine fungal communities associated with syconia of six species of Ficus and their pollinating wasps in lowland Panama. Diverse fungi were recovered from surface-sterilized flowers of all Ficus species, including gall- and seed flowers at four developmental stages. Fungal communities in syconia and on pollinating wasps were similar, dominated by diverse and previously unknown Saccharomycotina, and distinct from leaf- and stem endophyte communities in the same region. Before pollination, fungal communities were similar between gall- and seed flowers and among Ficus species. However, fungal communities differed significantly in flowers after pollination vs. before pollination, and between anciently diverged lineages of Ficus with active vs. passive pollination syndromes. Within groups of relatively closely related figs, there was little evidence for strict-sense host specificity between figs and particular fungal species. Instead, mixing of fungal communities among related figs, coupled with evidence for possible transfer by pollinating wasps, is consistent with recent suggestions of pollinator mixing within syconia. In turn, changes in fungal communities during fig development and ripening suggest an unexplored role of yeasts in the context of the fig–pollinator wasp mutualism.



Similar content being viewed by others
References
Agrawal AA, Ackerly DD et al (2007) Filling key gaps in population and community ecology. Front Ecol Environ 5:145–152
Altschul SF, Gish W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Arnold AE, Maynard Z et al (2000) Are tropical fungal endophytes hyperdiverse? Ecol Lett 3:267–274
Arnold AE, Maynard Z et al (2001) Fungal endophytes in dicotyledonous neotropical trees: patterns of abundance and diversity. Mycol Res 105:1502–1507
Arnold AE, Herre EA (2003) Canopy cover and leaf age affect colonization by tropical fungal endophytes: ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia 95:388–398
Arnold AE, Henk DA et al (2007) Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia 99:185–206
Arnold AE, Lutzoni F (2007) Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88:541–549
Arnold AE, Miadlikowska J et al (2009) A phylogenetic estimation of trophic transition networks for ascomycetous fungi: are lichens cradles of symbiotrophic fungal diversification? Syst Biol 58:283–297
Barash I, Manulis-Sasson S (2009) Recent evolution of bacterial pathogens: the gall-forming Pantoea agglomerans case. Annu Rev Phytopathol 47:133–152
Barker JSF (1992) Genetic variation in cactophilic Drosophila for oviposition on natural yeast substrates. Evolution 46:1070–1083
Baumann P, Baumann L et al (1995) Genetics, physiology, and evolutionary relationships of the genus Buchnera—intracellular symbionts of aphids. Annu Rev Microbiol 49:55–94
Berg CC (1989) Classification and distribution of Ficus. Experientia 45:605–611
Bronstein JL (1988) Mutualism, antagonism, and the fig–pollinator interaction. Ecology 69:1298–1302
Bronstein JL, Alarcon R et al (2006) The evolution of plant–insect mutualisms. New Phytol 172:412–428
Bronstein JL, Barbosa P (2002) Multi-trophic/multi-species mutualistic interactions: the role of non-mutualists in shaping and mediating mutualisms. In: Tscharntke T, Hawkins BA (eds) Multitrophic level interactions. Cambridge University Press, Cambridge, pp 44–66
Cairney JWG (1999) Intraspecific physiological variation: implications for understanding functional diversity in ectomycorrhizal fungi. Mycorrhiza 9:125–135
Cardoza YJ, Teal PEA et al (2003) Effect of peanut plant fungal infection on oviposition preference by Spodoptera exigua and on host-searching behavior by Cotesia marginiventris. Environ Entomol 32:970–976
Chen YC, Eisner JD et al (2001) Polymorphic internal transcribed spacer region 1 DNA sequences identify medically important yeasts. J Clin Microbiol 39:4042–4051
Clarke KR, Green RH (1988) Statistical design and analysis for a biological effects study. Mar Ecol Prog Ser 46:213–226
Clay, K. (1988) Fungal endophytes of grasses—a defensive mutualism between plants and fungi. Ecology 6910-16
Compton SG, Ellwood MDF et al (2000) The flight heights of chalcid wasps (Hymenoptera, Chalcidoidea) in a lowland Bornean rain forest: fig wasps are the high fliers. Biotropica 32:515–522
Currie CR, Scott JA et al (2003) Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 423:461–461
Daisy BH, Strobel GA et al (2002) Naphthalene, an insect repellent, is produced by Muscodor vitigenus, a novel endophytic fungus. Microbiology 148:3737–3741
Doster MA, Michailides TJ et al (1996) Aspergillus species and mycotoxins in figs from California orchards. Plant Dis 80:484–489
Dunn DW, Segar ST et al (2008) A role for parasites in stabilizing the fig–pollinator mutualism. PLoS Biol 6:490–496
Dunn DW, Yu DW et al (2008) Longevity, early emergence and body size in a pollinating fig wasp—implications for stability in a fig–pollinator mutualism. J Anim Ecol 77(5):927–935
Fisher RA, Corbet AS et al (1943) The relation between the number of species and the number of individuals in a random sample of an animal population. J Anim Ecol 12:42–58
Feldman TS, O’Brien H et al (2008) Moth dispersal of mycoparasites and endophytes associated with Claviceps paspali and the grass Paspalum (Poaceae). Microb Ecol 56:742–750
Gallery RE, Dalling JW et al (2007) Diversity, host affinity, and distribution of seed-infecting fungi: a case study with Cecropia. Ecology 88:582–588
Ganeshaiah KN, Kathuria P et al (1995) Evolution of style-length variability in figs and optimization of ovipositor length in their pollinator wasps—a coevolutionary model. J Genet 74:25–39
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gehring C, Bennett A (2009) Mycorrhizal fungal–plant–insect interactions: the importance of a community approach. Environ Entomol 38:93–102
Gibson C, Hunter M (2009) Inherited fungal and bacterial endosymbionts of a parasitic wasp and its cockroach host. Microb Ecol 57:542–549
Goodrich KR, Zjhra ML et al (2006) When flowers smell fermented: the chemistry and ontogeny of yeasty floral scent in pawpaw (Asimina triloba: Annonaceae). Int J Plant Sci 167:33–46
Grison-Pige L, Hossaert-McKey M et al (2002) Fig volatile compounds—a first comparative study. Phytochemistry 61:61–71
Hamanaka D, Norimura N et al (2010) Surface decontamination of fig fruit by combination of infrared radiation heating with ultraviolet irradiation. Food Control 22:375–380
Hammer O, Harper DAT et al (2001) PAST: Paleontological Statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Herre EA (1993) Population structure and the evolution of virulence in nematode parasites of fig wasps. Science 259:1442–1445
Herre EA (1995) Factors affecting the evolution of virulence: nematode parasites of fig wasps as a case study. Parasitology 111:S179–S191
Herre EA, Knowlton N et al (1999) The evolution of mutualisms: exploring the paths between conflict and cooperation. Trends Ecol Evol 14:49–53
Herre EA, West SA (1997) Conflict of interest in a mutualism: documenting the elusive fig wasp–seed trade-off. Proc R Soc Lond B Biol Sci 264:1501–1507
Higgins KL, Coley PD et al (2011) Culturing and direct PCR suggest prevalent host generalism among diverse fungal endophytes of tropical forest grasses. Mycologia 103:247–260
Hoffman MT, Arnold AE (2010) Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Appl Environ Microbiol 76:4063–4075
Hossaert-Mckey M, Gibernau M et al (1994) Chemosensory attraction of fig wasps to substances produced by receptive figs. Entomol Exp Appl 70:185–191
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Jallow MFA, Dugassa-Gobena D et al (2008) Influence of an endophytic fungus on host plant selection by a polyphagous moth via volatile spectrum changes. Arthropod-Plant Interactions 2:53–62
Jander KC, Herre EA (2010) Host sanctions and pollinator cheating in the fig tree–fig wasp mutualism. Proc R Soc Lond B Biol Sci 277:1481–1488
Janzen DH (1966) Coevolution of mutualism between ants and Acacias in Central America. Evolution 20:249
Janzen DH (1979) How to be a fig. Annu Rev Ecol Syst 10:13–51
Jousselin E, Hossaert-McKey M et al (2003) Why do fig wasps actively pollinate monoecious figs? Oecologia 134:381–387
Katoh K, Misawa K et al (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066
Kjellberg F, Doumesche B et al (1988) Longevity of a fig wasp (Blastophaga-psenes). Proc K Ned Akad Wet C 91:117–122
Leigh EG, Rand AS et al (1996) The ecology of a tropical forest: seasonal rhythms and long term changes. Smithsonian Institute, Washington
Lopez-Vaamonde C, Winkström N et al (2009) Molecular dating and biogeography of fig-pollinating wasps. Mol Phylogenet Evol 52:715–726
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235
Machado CA, Jousselin E et al (2001) Phylogenetic relationships, historical biogeography and character evolution of fig-pollinating wasps. Proc R Soc Lond B Biol Sci 268:685–694
Machado CA, Robbins N et al (2005) Critical review of host specificity and its coevolutionary implications in the fig/fig wasp mutualism. Proc Natl Acad Sci U S A 102:6558–6565
Maddison WP, Maddison DR (2009) Mesquite: a modular system for evolutionary analysis
Miller MW, Phaff HJ (1962) Successive microbial populations in Calimyrna figs. Appl Microbiol 10(5):394–400
Mrak EM, Phaff HJ et al (1942) Yeasts occurring in souring figs. J Bacteriol 44:441–450
Marussich WA, Machado CA (2007) Host-specificity and coevolution among pollinating and nonpollinating New World fig wasps. Mol Ecol 16:1925–1946
Mooney KA, Mandal K (2010) Competition hierarchies among ants and predation by birds jointly determine the strength of multi-species ant–aphid mutualisms. Oikos 119:874–882
Oliver KM, Degnan PH et al (2009) Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325:992–994
Omacini M, Chaneton EJ et al (2001) Symbiotic fungal endophytes control insect host–parasite interaction webs. Nature 409:78–81
Parlade J, Hortal S et al (2011) Intraspecific variability of Lactarius deliciosus isolates: colonization ability and survival after cold storage. Mycorrhiza 21:393–401
Pellmyr O, Huth CJ (1994) Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372:257–260
Pitzschke A, Hirt H (2010) New insights into an old story: Agrobacterium-induced tumour formation in plants by plant transformation. EMBO J 29:1021–1032
Posada D (2006) ModelTest Server: a web-based tool for the statistical selection of models of nucleotide substitution online. Nucleic Acids Res 34:W700–W703
Raguso RA (2004) Why are some floral nectars scented? Ecology 85:1486–1494
Rohfritsch O (2008) Plants, gall midges, and fungi: a three-component system. Entomol Exp Appl 128:208–216
Rønsted N, Weiblen G et al (2005) 60 million years of co-divergence in the fig–wasp symbiosis. Proc R Soc Lond B Biol Sci 272:2593–2599
Rudgers JA, Gardener MC (2004) Extrafloral nectar as a resource mediating multispecies interactions. Ecology 85:1495–1502
Sanchez F, Korine C et al (2006) Ethanol and methanol as possible odor cues for Egyptian fruit bats (Rousettus aegyptiacus). J Chem Ecol 32:1289–1300
Stamatakis A (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Suryanarayanan TS, Vijaykrishna D (2001) Fungal endophytes of aerial roots of Ficus benghalensis. Fungal Divers 8:155–161
U'Ren JM, Dalling JW et al (2009) Diversity and evolutionary origins of fungi associated with seeds of a neotropical pioneer tree: a case study for analysing fungal environmental samples. Mycol Res 113:432–449
U'Ren JM, Lutzoni F et al (2010) Community analysis reveals close affinities between endophytic and endolichenic fungi in mosses and lichens. Microb Ecol 60:340–353
Van Bael SA, Fernandez-Marin H et al (2009) Two fungal symbioses collide: endophytic fungi are not welcome in leaf-cutting ant gardens. Proc R Soc Lond B Biol Sci 276:2419–2426
van Noort S, Ware AB et al (1989) Pollinator-specific volatile attractants released from the figs of Ficus Burtt-davyi. S Afr J Sci 85:323–324
Verkerke W (1986) Anatomy of Ficus ottoniifolia (Moraceae) syconia and its role in the fig–fig wasp symbiosis. Proc K Ned Akad Wet 89:443–469
Vidal S (1996) Changes in suitability of tomato for whiteflies mediated by a non-pathogenic endophytic fungus. Entomol Exp Appl 80:272–274
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Wang HK, Hyde KD et al (2008) Fungal diversity on fallen leaves of Ficus in northern Thailand. J Zhejiang Univ Sci B 9:835–841
Wang GQ, Wei SG et al (2009) Six new eriophyoid mites (Acari: Eriophyoidea) associated with Ficus spp. (Moraceae) from China. Zootaxa 2201:49–62
Ware AB, Kaye PT et al (1993) Fig volatiles—their role in attracting pollinators and maintaining pollinator specificity. Plant Syst Evol 186:147–156
Way MJ (1963) Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol 8:307–344
Weiblen GD (2002) How to be a fig wasp. Annu Rev Entomol 47:299–330
West SA, Herre EA (1994) The ecology of the New-World fig-parasitizing wasps Idarnes and implications for the evolution of the fig–pollinator mutualism. Proc R Soc Lond B Biol Sci 258:67–72
Acknowledgments
We gratefully acknowledge the National Science Foundation for supporting this research (IOB-062492 to AEA and an NSF Graduate Research Fellowship to EOM) as well as the Smithsonian Institute (Predoctoral Fellowship to EOM). We thank the Smithsonian Tropical Research Institute for logistical support and the government of Panama for permission to carry out this research. We are grateful to J. Hackett and A. Gomez for technical assistance, W. Marussich for collection of fig wasps, and J. U’Ren and V. Martinson for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Locations of trees sampled in Barro Colorado National Monument, Panama. 1 F. insipida, 2 F. maxima, 3 F. triangle, 4 F. costaricana, 5 F. popenoei, and 6 F. obtusifolia. Physically proximate trees harbored communities that were actually significantly less similar (comparison among trees 1–4; averaged JGR = 0.6891) than trees at further distances (trees 5–6 compared to trees 1–4; averaged JGR = 0.9804) (JPEG 1576 kb)
Supplemental Figure 2
Most likely tree resulting from RAxML analyses of the 5.8S and partial LSU, including results of maximum likelihood bootstrap (≥70 %; above branches) and Bayesian posterior probabilities (≥90 %, below branches), revealing the phylogenetic placement of fungal OTU recovered from figs in six species of Ficus in Panama. Tree was used for UniFrac analyses; clades are not collapsed, such that the position of each sequence can be examined. Leucosporidium antarcticum was included as an outgroup. Black bars indicate OTU based on 95 % sequence similarity (JPEG 5775 kb)
Supplemental Figure 3
OTU accumulation, 95 % confidence intervals, and bootstrap estimates of richness based on ITS-LSU OTU (95 % sequence similarity) for fungal communities sampled from each of six Ficus species (JPEG 2737 kb)
Supplemental Figure 4
OTU accumulation for fungi recovered from pollinating fig wasps, 95 % confidence intervals, and bootstrap estimates of richness based on the survey of six species of wasps in lowland Panama (JPEG 2618 kb)
Supplemental Figure 5
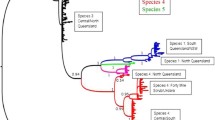
Most likely tree resulting from RAxML analyses of the 5.8S and partial LSU, including results of maximum likelihood bootstrap (≥70 %; above branches), revealing the phylogenetic placement of fungal OTU recovered from figs in six species of Ficus and their associated pollinating fig wasps in Panama. L. antarcticum was included as an outgroup. Black bars indicate OTU based on 95 % sequence similarity for Ficus sequences and red bars indicate sequences from their pollinating fig wasps (JPEG 4531 kb)
Supplemental Figure 6
Non-metric multidimensional scaling (NMDS) analysis of gall- and seed-flower fungal communities. Additional analysis shown in upper right corner was assessed by ANOSIM with the Morisita index based on non-singleton OTU (JPEG 241 kb)
Rights and permissions
About this article
Cite this article
Martinson, E.O., Herre, E.A., Machado, C.A. et al. Culture-Free Survey Reveals Diverse and Distinctive Fungal Communities Associated with Developing Figs (Ficus spp.) in Panama. Microb Ecol 64, 1073–1084 (2012). https://doi.org/10.1007/s00248-012-0079-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0079-x