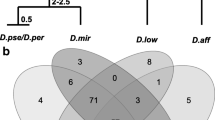
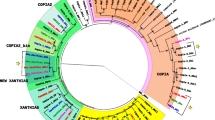
Abstract
Interspecies divergence of orthologous transposable element remnants is often assumed to be simply due to genetic drift of neutral mutations that occurred after the divergence of the species. However, divergence may also be affected by other factors, such as variation in the mutation rate, ancestral polymorphisms, or selection. Here we attempt to determine the impact of these forces on divergence of three classes of sites that are often assumed to be selectively unconstrained (INE-1 TE remnants, sites within short introns, and fourfold degenerate sites) in two different pairwise comparisons of Drosophila (D. melanogaster vs. D. simulans and D. simulans vs. D. sechellia). We find that divergence of these three classes of sites is strongly influenced by the recombination environment in which they are located, and this is especially true for the closer D. simulans vs. D. sechellia comparison. We suggest that this is mainly a result of the contribution of ancestral polymorphisms in different recombination regions. We also find that intergenic INE-1 elements are significantly more diverged than intronic INE-1 in both pairwise comparisons, implying the presence of either negative selection or lower mutation rates in introns. Furthermore, we show that substitution rates in INE-1 elements are not associated with the length of the noncoding sequence in which they are located, suggesting that reduced divergence in long noncoding sequences is not due to reduced mutation rates in these regions. Finally, we show that GC content for each site within INE-1 sequences has evolved toward an equilibrium value (∼33%) since insertion.




Similar content being viewed by others
References
Akashi H (1995) Inferring weak selection from patterns of polymorphism and divergence at “silent” sites in Drosophila DNA. Genetics 139:1067–1076
Aquado CF, Begun DJ, Kindahl EC (1994) Selection, recombination, and DNA polymorphism in Drosophila. In: Golding B (ed) Non-neutral evolution: theories and molecular data. Chapman and Hall, London, pp 46–56
Begun DJ, Aquadro CF (1992) Levels of naturally occurring DNA polymorphism correlate with recombination rates in D. melanogaster. Nature 356:519–520
Berg DE, Howe MM (1989) Mobile DNA. ASM Press, Herndon, VA
Betancourt AJ, Presgraves DC (2002) Linkage limits the power of natural selection in Drosophila. Proc Natl Acad Sci USA 99:13616–13620
Bray N, Pachter L (2004) MAVID: constrained ancestral alignment of multiple sequences. Genome Res 14:693–699
Charlesworth B (1996) Background selection and patterns of genetic diversity in Drosophila melanogaster. Genet Res 68:131–149
Charlesworth B, Langley CH (1989) The population genetics of Drosophila transposable elements. Annu Rev Genet 23:251–287
Charlesworth B, Lapid A (1989) A study of ten transposable elements on X chromosomes from a population of Drosophila melanogaster. Genet Res 54:113–125
Charlesworth B, Lapid A, Canada D (1992) The distribution of transposable elements within and between chromosomes in a population of Drosophila melanogaster. I. Element frequencies and distribution. Genet Res 60:103–114
Deaconescu AM, Chambers AL, Smith AJ, et al. (2006) Structural basis for bacterial transcription-coupled DNA repair. Cell 124:507–520
Deininger PL, Batzer MA (2002) Mammalian retroelements. Genome Res 12:1455–1465
Deininger PL, Moran JV, Batzer MA, Kazazian HH Jr (2003) Mobile elements and mammalian genome evolution. Curr Opin Genet Dev 13:651–658
Felsenstein J (1974) The evolutionary advantage of recombination. Genetics 78:737–756
Gaffney DJ, Keightley PD (2006) Genomic selective constraints in murid noncoding DNA. PLoS Genet 2:e204
Haddrill PR, Charlesworth B, Halligan DL, Andolfatto P (2005) Patterns of intron sequence evolution in Drosophila are dependent upon length and GC content. Genome Biol 6:R67
Haddrill PR, Halligan DL, Tomaras D, Charlseworth B (2007) Reduced efficacy of selection in regions of the Drosophila genome that lack crossing over. Genome Biol 8:R18
Halligan DL, Keightley PD (2006) Ubiquitous selective constraints in the Drosophila genome revealed by a genome-wide interspecies comparison. Genome Res 16:875–884
Hardison RC, Roskin KM, Yang S, et al. (2003) Covariation in frequencies of substitution, deletion, transposition, and recombination during eutherian evolution. Genome Res 13:13–26
Hellmann I, Ebersberger I, Ptak SE, Pääbo S, Przeworski M (2003) A neutral explanation for the correlation of diversity with recombination rates in humans. Am J Hum Genet 72:1527–1535
Hey J, Kliman RM (2002) Interactions between natural selection, recombination and gene density in the genes of Drosophila. Genetics 160:595–608
Hill WG, Robertson A (1966) The effect of linkage on limits to artificial selection. Genet Res 8:269–294
Jakubczak JL, Xiong Y, Eickbush TH (1990) Type I (R1) and type II (R2) ribosomal DNA insertions of Drosophila melanogaster are retrotransposable elements closely related to those of Bombyx mori. J Mol Biol 212:37–52
Jensen MA, Charlesworth B, Kreitman M (2002) Patterns of genetic variation at a chromosome 4 locus of Drosophila melanogaster and D. simulans. Genetics 160:493–507
Jordan IK, Rogozin IB, Glazko GV, Koonin EV (2003) Origin of a substantial fraction of human regulatory sequences from transposable elements. Trends Genet 19:68–72
Kapitonov VV, Jurka J (1999) DNAREP1_DM. Repbase update release 3.4. Available at: http://www.girinst.org/Repbase_Updata.html
Kapitonov VV, Jurka J (2003) Molecular paleontology of transposable elements in the Drosophila melanogaster genome. Proc Natl Acad Sci USA 100:6569–6574
Kaplan NL, Hudson RR, Langley CH (1989) The “hitch-hiking effect” revisited. Genetics 123(4):887–899
Kazazian HH (2004) Mobile elements: drivers of genome evolution. Science 303(5664):1626–1632
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kirby DA, Muse SV, Stephan W (1995) Maintenance of pre-mRNA secondary structure by epistatic selection. Proc Natl Acad Sci USA 92:9047–9051
Kliman RM, Hey J (1993) Reduced natural selection associated with low recombination in Drosophila melanogaster. Mol Biol Evol 10:1239–1258
LePage DF, Church DM, Millie E, Hassold TJ, Conlon RA (2000) Rapid generation of nested chromosomal deletions on mouse chromosome 2. Proc Natl Acad Sci USA 97:10471–10476
Lercher MJ, Hurst LD (2002) Human SNP variability and mutation rate are higher in regions of high recombination. Trends Genet 18:337–340
Marais G, Domazet-Loso T, Tautz D, Charlesworth B (2004) Correlated evolution of synonymous and nonsynonymous sites in Drosophila. J Mol Evol 59:771–779
Maynard-Smith J, Haigh J (1974) The hitch-hiking effect of a favorable gene. Genet Res 23:23–35
McDonald JF (1993) Evolution and consequences of transposable elements. Curr Opin Genet Dev 3:855–864
McVean GA, Vieira J (2001) Inferring parameters of mutation, selection and demography from patterns of synonymous site evolution in Drosophila. Genetics 157:245–257
Moriyama EN, Powell JR (1996) Intraspecific nuclear DNA variation in Drosophila. Mol Biol Evol 13:261–277
Moriyama EN, Powell JR (1997) Synonymous substitution rates in Drosophila: mitochondrial versus nuclear genes. J Mol Evol 45:378–391
Mozer BA, Benzer S (1994) Ingrowth by photoreceptor axons induces transcription of a retrotransposon in the developing Drosophila brain. Development 120:1049–1058
Pardue ML, DeBaryshe PG (2003) Retrotransposons provide an evolutionarily robust non-telomerase mechanism to maintain telomeres. Annu Rev Genet 37:485–511
Petrov DA, Hartl DL (1999) Patterns of nucleotide substitution in Drosophila and mammalian genomes. Proc Natl Acad Sci USA 96:1475–1479
Presgraves DC (2005) Recombination enhances protein adaptation in Drosophila melanogaster. Curr Biol 15:1651–1656
Pyatkov KI, Shostak NG, Zelentsova ES, et al. (2002) Penelope retroelements from Drosophila virilis are active after transformation of Drosophila melanogaster. Proc Natl Acad Sci USA 99:16150–16155
Quesneville H, Bergman CM, Andrieu O, et al. (2005) Combined evidence annotation of transposable elements in genome sequences. PLoS Comput Biol 1:166–175
Shapiro JA, Huang W, Zhang C, Hubisz MJ, Lu J, Turissini DA, Fang S, Wang HY, Hudson RR, Nielsen R, Chen Z, Wu CI (2007) Adaptive genic evolution in the Drosophila genomes, Proc Natl Acad Sci USA 104(7):2271–2276
Singh ND, Petrov DA (2004) Rapid sequence turnover at an intergenic locus in Drosophila. Mol Biol Evol 21:670–680
Singh ND, Arndt PF, Petrov DA (2005a) Genomic heterogeneity of background substitutional patterns in Drosophila melanogaster. Genetics 169:709–722
Singh ND, Davis JC, Petrov DA (2005b) Codon bias and noncoding GC content correlate negatively with recombination rate on the Drosophila X chromosome. J Mol Evol 61:315–324
Slawson EE, Shaffer CD, Malone CD, et al. (2006) Comparison of dot chromosome sequences from D. melanogaster and D. virilis reveals an enrichment of DNA transposon sequences in heterochromatic domains. Genome Biol 7:R15
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
van de Lagemaat LN, Landry JR, Mager DL, Medstrand P (2003) Transposable elements in mammals promote regulatory variation and diversification of genes with specialized functions. Trends Genet 19:530–536
Venter JC, Adams MD, Myers EW, et al. (2001) The sequence of the human genome. Science 291:1304–1351
Wang J, Keightley PD, Johnson T (2006) MCALIGN2: faster, accurate global pairwise alignment of non-coding DNA sequences based on explicit models of indel evolution. BMC Bioinform 7:292
Waterston RH, Lindblad-Toh K, Birney E, et al. (2002) Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562
White SE, Habera LF, Wessler SR (1994) Retrotransposons in the flanking regions of normal plant genes: a role for copia-like elements in the evolution of gene structure and expression. Proc Natl Acad Sci USA 91:11792–11796
Yang H-P, Hung T-L, You T-L, Yang T-H (2006) Genomewide comparative analysis of the highly abundant transposable element DINE-1 suggests a recent transpositional burst in Drosophila yakuba. Genetics 173(1):189–196
Yi S, Summers TJ, Pearson NM, Li W-H (2004) Recombination has little effect on the rate of sequence divergence in pseudoautosomal boundary 1 among humans and great apes. Genome Res 14:37–43
Acknowledgments
We are grateful to the Genome Sequence Center, WUSTL School of Medicine, the Broad Institute of MIT and Harvard, and the Berkeley Drosophila Genome Project for providing the genome sequences we analyzed in this study. We also thank Flybase and NCBI for providing genome annotation data. We thank Toby Johnson, Daniel Gaffney, and Brian Charlesworth for helpful comments. J.W. was supported by the Dorothy Hodgkin Postgraduate Studentship Award. Funding for D.L.H. was provided by the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Electronic supplementary material
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, J., Keightley, P.D. & Halligan, D.L. Effect of Divergence Time and Recombination Rate on Molecular Evolution of Drosophila INE-1 Transposable Elements and Other Candidates for Neutrally Evolving Sites. J Mol Evol 65, 627–639 (2007). https://doi.org/10.1007/s00239-007-9028-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-007-9028-6