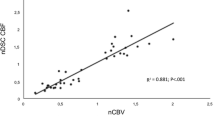
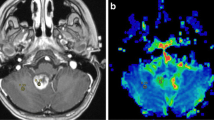
Abstract
Purpose
Dynamic susceptibility contrast (DSC) and arterial spin labeling (ASL) perfusion MRI are applied in pediatric brain tumor grading, but their value for clinical daily practice remains unclear. We explored the ability of ASL and DSC to distinguish low- and high-grade lesions, in an unselected cohort of pediatric cerebral tumors.
Methods
We retrospectively compared standard perfusion outcomes including blood volume, blood flow, and time parameters from DSC and ASL at 1.5T or 3T MRI scanners of 46 treatment-naive patients by drawing ROI via consensus by two neuroradiologists on the solid portions of every tumor. The discriminant abilities of perfusion parameters were evaluated by receiver operating characteristic (ROC) over the entire cohort and depending on the tumor location and the magnetic field.
Results
ASL and DSC parameters showed overall low to moderate performances to distinguish low- and high-grade tumors (area under the curve: between 0.548 and 0.697). Discriminant abilities were better for tumors located supratentorially (AUC between 0.777 and 0.810) than infratentorially, where none of the metrics reached significance. We observed a better differentiation between low- and high-grade cancers at 3T than at 1.5-T. For infratentorial tumors, time parameters from DSC performed better than the commonly used metrics (AUC ≥ 0.8).
Conclusion
DSC and ASL show moderate abilities to distinguish low- and high-grade brain tumors in an unselected cohort. Absolute value of K2, TMAX, tMIP, and normalized value of TMAX of the DSC appear as an alternative to conventional parameters for infratentorial tumors. Three Tesla evaluation should be favored over 1.5-Tesla.




Similar content being viewed by others
Data availability
Data are available under conditions
References
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2017) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncol 19:v1–v88. https://doi.org/10.1093/neuonc/nox158
Borja MJ, Plaza MJ, Altman N, Saigal G (2013) Conventional and advanced MRI features of pediatric intracranial tumors: supratentorial tumors. Am J Roentgenol 200:W483–W503. https://doi.org/10.2214/AJR.12.9724
Porto L, Jurcoane A, Schwabe D, Hattingen E (2014) Conventional magnetic resonance imaging in the differentiation between high and low-grade brain tumours in paediatric patients. Eur J Paediatr Neurol 18:25–29. https://doi.org/10.1016/j.ejpn.2013.07.004
Poretti A, Meoded A, Huisman TAGM (2012) Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging 35:32–47. https://doi.org/10.1002/jmri.22722
Sui Y, Wang H, Liu G, Damen FW, Wanamaker C, Li Y, Zhou XJ (2015) Differentiation of low- and high-grade pediatric brain tumors with high b -value diffusion-weighted MR imaging and a fractional order calculus model. Radiology 277:489–496. https://doi.org/10.1148/radiol.2015142156
Porto L, Jurcoane A, Schwabe D, Kieslich M, Hattingen E (2013) Differentiation between high and low grade tumours in paediatric patients by using apparent diffusion coefficients. Eur J Paediatr Neurol 17:302–307. https://doi.org/10.1016/j.ejpn.2012.12.002
Vicente J, Fuster-Garcia E, Tortajada S, García-Gómez JM, Davies N, Natarajan K, Wilson M, Grundy RG, Wesseling P, Monleón D, Celda B, Robles M, Peet AC (2013) Accurate classification of childhood brain tumours by in vivo 1H MRS – a multi-centre study. Eur J Cancer 49:658–667. https://doi.org/10.1016/j.ejca.2012.09.003
Öz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dinçer A, Dydak U, Emir UE, Frahm J, González RG, Gruber S, Gruetter R, Gupta RK, Heerschap A, Henning A, Hetherington HP, Howe FA, Hüppi PS, Hurd RE, Kantarci K, Klomp DWJ, Kreis R, Kruiskamp MJ, Leach MO, Lin AP, Luijten PR, Marjańska M, Maudsley AA, Meyerhoff DJ, Mountford CE, Nelson SJ, Pamir MN, Pan JW, Peet AC, Poptani H, Posse S, Pouwels PJW, Ratai EM, Ross BD, Scheenen TWJ, Schuster C, Smith ICP, Soher BJ, Tkáč I, Vigneron DB, Kauppinen RA, For the MRS Consensus Group (2014) Clinical proton MR spectroscopy in central nervous system disorders. Radiology 270:658–679. https://doi.org/10.1148/radiol.13130531
Koob M, Girard N, Ghattas B, Fellah S, Confort-Gouny S, Figarella-Branger D, Scavarda D (2016) The diagnostic accuracy of multiparametric MRI to determine pediatric brain tumor grades and types. J Neurooncol 127:345–353. https://doi.org/10.1007/s11060-015-2042-4
Tzika A, Astrakas L, Zarifi M, Petridou N, Young-Poussaint T, Goumnerova L, Zurakowski D, Anthony D, Black P (2003) Multiparametric MR assessment of pediatric brain tumors. Neuroradiology 45:1–10. https://doi.org/10.1007/s00234-002-0865-0
Poussaint TY, Rodriguez D (2006) Advanced neuroimaging of pediatric brain tumors: MR diffusion, MR perfusion, and MR spectroscopy. Neuroimaging Clin N Am 16:169–192. https://doi.org/10.1016/j.nic.2005.11.005
Calmon R, Puget S, Varlet P, Dangouloff-Ros V, Blauwblomme T, Beccaria K, Grevent D, Sainte-Rose C, Castel D, Debily MA, Dufour C, Bolle S, Dhermain F, Saitovitch A, Zilbovicius M, Brunelle F, Grill J, Boddaert N (2018) Cerebral blood flow changes after radiation therapy identifies pseudoprogression in diffuse intrinsic pontine gliomas. Neuro-Oncol 20:994–1002. https://doi.org/10.1093/neuonc/nox227
Ball WS, Holland SK (2001) Perfusion imaging in the pediatric patient. Magn Reson Imaging Clin N Am 9(207–230):ix
Ho CY, Cardinal JS, Kamer AP, Kralik SF (2015) Relative cerebral blood volume from dynamic susceptibility contrast perfusion in the grading of pediatric primary brain tumors. Neuroradiology 57:299–306. https://doi.org/10.1007/s00234-014-1478-0
Ho CY, Cardinal JS, Kamer AP, Lin C, Kralik SF (2016) Contrast leakage patterns from dynamic susceptibility contrast perfusion MRI in the grading of primary pediatric brain tumors. Am J Neuroradiol 37:544–551. https://doi.org/10.3174/ajnr.A4559
Cha S (2006) Dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in pediatric patients. Neuroimaging Clin N Am 16:137–147. https://doi.org/10.1016/j.nic.2005.11.006
Dallery F, Bouzerar R, Michel D, Attencourt C, Promelle V, Peltier J, Constans JM, Balédent O, Gondry-Jouet C (2017) Perfusion magnetic resonance imaging in pediatric brain tumors. Neuroradiology 59:1143–1153. https://doi.org/10.1007/s00234-017-1917-9
Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M (2015) A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology 57:1181–1202. https://doi.org/10.1007/s00234-015-1571-z
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJP, Wang DJJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia: recommended implementation of ASL for clinical applications. Magn Reson Med 73:102–116. https://doi.org/10.1002/mrm.25197
Yeom KW, Mitchell LA, Lober RM, Barnes PD, Vogel H, Fisher PG, Edwards MS (2014) Arterial spin-labeled perfusion of pediatric brain tumors. Am J Neuroradiol 35:395–401. https://doi.org/10.3174/ajnr.A3670
Morana G, Piccardo A, Tortora D, Puntoni M, Severino M, Nozza P, Ravegnani M, Consales A, Mascelli S, Raso A, Cabria M, Verrico A, Milanaccio C, Rossi A (2017) Grading and outcome prediction of pediatric diffuse astrocytic tumors with diffusion and arterial spin labeling perfusion MRI in comparison with 18F–DOPA PET. Eur J Nucl Med Mol Imaging 44:2084–2093. https://doi.org/10.1007/s00259-017-3777-2
Kikuchi K, Hiwatashi A, Togao O, Yamashita K, Yoshimoto K, Mizoguchi M, Suzuki SO, Iwaki T, Suzuki Y, Honda H (2017) Correlation between arterial spin-labeling perfusion and histopathological vascular density of pediatric intracranial tumors. J Neurooncol 135:561–569. https://doi.org/10.1007/s11060-017-2604-8
Dangouloff-Ros V, Deroulers C, Foissac F, Badoual M, Shotar E, Grévent D, Calmon R, Pagès M, Grill J, Dufour C, Blauwblomme T, Puget S, Zerah M, Sainte-Rose C, Brunelle F, Varlet P, Boddaert N (2016) Arterial spin labeling to predict brain tumor grading in children: correlations between histopathologic vascular density and perfusion MR imaging. Radiology 281:553–566. https://doi.org/10.1148/radiol.2016152228
Morana G, Tortora D, Staglianò S, Nozza P, Mascelli S, Severino M, Piatelli G, Consales A, Lequin M, Garrè ML, Rossi A (2018) Pediatric astrocytic tumor grading: comparison between arterial spin labeling and dynamic susceptibility contrast MRI perfusion. Neuroradiology 60:437–446. https://doi.org/10.1007/s00234-018-1992-6
Novak J, Withey SB, Lateef S, MacPherson L, Pinkey B, Peet AC (2019) A comparison of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast MRI with and without contrast agent leakage correction in paediatric brain tumours. Br J Radiol 92:20170872. https://doi.org/10.1259/bjr.20170872
Vidyasagar R, Abernethy L, Pizer B, Avula S, Parkes LM (2016) Quantitative measurement of blood flow in paediatric brain tumours—a comparative study of dynamic susceptibility contrast and multi time-point arterial spin labelled MRI. Br J Radiol 89:20150624. https://doi.org/10.1259/bjr.20150624
European Society of Radiology (ESR) (2020) ESR statement on the validation of imaging biomarkers. Insights Imaging 11:76. https://doi.org/10.1186/s13244-020-00872-9
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol (Berl) 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Welker K, Boxerman J, Kalnin A, Kaufmann T, Shiroishi M, Wintermark M, for the American Society of Functional Neuroradiology MR Perfusion Standards and Practice Subcommittee of the ASFNR Clinical Practice Committee (2015) ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. Am J Neuroradiol 36:E41–E51. https://doi.org/10.3174/ajnr.A4341
Tanaka Y, Nagaoka T, Nair G, Ohno K, Duong TQ (2011) Arterial spin labeling and dynamic susceptibility contrast CBF MRI in postischemic hyperperfusion, hypercapnia, and after mannitol injection. J Cereb Blood Flow Metab 31:1403–1411. https://doi.org/10.1038/jcbfm.2010.228
Hales PW, d’Arco F, Cooper J et al (2019) Arterial spin labelling and diffusion-weighted imaging in paediatric brain tumours. NeuroImage Clin 22:101696. https://doi.org/10.1016/j.nicl.2019.101696
Leon SP, Folkerth RD, Black PM (1996) Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 77:362–372. https://doi.org/10.1002/(SICI)1097-0142(19960115)77:2<362::AID-CNCR20>3.0.CO;2-Z
Plate KH, Scholz A, Dumont DJ (2012) Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol (Berl) 124:763–775. https://doi.org/10.1007/s00401-012-1066-5
Straka M, Albers GW, Bammer R (2010) Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 32:1024–1037. https://doi.org/10.1002/jmri.22338
Law M, Young R, Babb J, Pollack E, Johnson G (2007) Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol 28:761–766
Delgado AF, De Luca F, Hanagandi P et al (2018) Arterial spin-labeling in children with brain tumor: a meta-analysis. Am J Neuroradiol 39(8):1536–1542. https://doi.org/10.3174/ajnr.A5727
Gevers S, van Osch MJ, Bokkers RP et al (2011) Intra- and multicenter reproducibility of pulsed, continuous and pseudo-continuous arterial spin labeling methods for measuring cerebral perfusion. J Cereb Blood Flow Metab 31:1706–1715. https://doi.org/10.1038/jcbfm.2011.10
Kaisti KK, Långsjö JW, Aalto S, et al. (2003) Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans: Anesthesiology 99:603–613. https://doi.org/10.1097/00000542-200309000-00015
Bauchet L, Rigau V, Mathieu-Daudé H, Fabbro-Peray P, Palenzuela G, Figarella-Branger D, Moritz J, Puget S, Bauchet F, Pallusseau L, Duffau H, Coubes P, Trétarre B, Labrousse F, Dhellemmes P, Société Française de Neurochirurgie Pédiatrique, Société Française de Neurochirurgie, Société Française de Neuropathologie, Association des Neuro-Oncologues d’Expression Française (2009) Clinical epidemiology for childhood primary central nervous system tumors. J Neurooncol 92:87–98. https://doi.org/10.1007/s11060-008-9740-0
Author information
Authors and Affiliations
Contributions
Benoit Testud: conception and design of the study, acquired and analyzed the data, drafted the manuscript and the figures. Gilles Brun: conception and design of the study, drafted the manuscript and the figures. Arthur Varoquaux: conception and design of the study, analyzed the data. Jean-François Hak: conception and design of the study. Romain Appay: conception and design of the study. Arnaud Le Troter: analyzed the data. Nadine Girard: conception and design of the study, acquired the data, drafted the manuscript and the figures. Jan-Patrick Stellmann: conception and design of the study, analyzed the data, drafted the manuscript and the figures.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Testud, B., Brun, G., Varoquaux, A. et al. Perfusion-weighted techniques in MRI grading of pediatric cerebral tumors: efficiency of dynamic susceptibility contrast and arterial spin labeling. Neuroradiology 63, 1353–1366 (2021). https://doi.org/10.1007/s00234-021-02640-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02640-y