Abstract
Purpose
Adverse drug events (ADE) are among the leading causes of morbidity and hospitalization. This review analyzes risk factors for ADE, particularly their categorizations and association patterns, the prevalence, severity, and preventability of ADE, and method characteristics of reviewed studies.
Methods
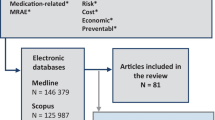
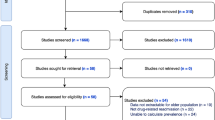
Literature search was conducted via PubMed, Science Direct, CINAHL, and MEDLINE. A review was conducted of research articles that reported original data about specific risk factors for ADE since 2000. Data analyses were performed using Excel and R.
Results
We summarized 211 risk factors for ADE, and grouped them into five main categories: patient-, disease-, medication-, health service-, and genetics-related. Among them, medication- and disease-related risk factors were most frequently studied. We further classified risk factors within each main category into subtypes. Among them, polypharmacy, age, gender, central nervous system agents, comorbidity, service utilization, inappropriate use/change use of drugs, cardiovascular agents, and anti-infectives were most studied subtypes. An association analysis of risk factors uncovered many interesting patterns. The median prevalence, preventability, and severity rate of reported ADE was 19.5% (0.29%~86.2%), 36.2% (2.63%~91%), and 16% (0.01%~47.4%), respectively.
Conclusions
This review introduced new categories and subtypes of risk factors for ADE. The broad and in-depth coverage of risk factors and their association patterns elucidate the complexity of risk factor analysis. Managing risk factors for ADE is crucial for improving patient safety, particularly for the elderly, comorbid, and polypharmacy patients. Some under-explored risk factors such as genetics, mental health and wellness, education, lifestyle, and physical environment invite future research.


Similar content being viewed by others
References
Rozich JD, Haraden CR, Resar RK (2003) Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care 12(>3):194–200. https://doi.org/10.1136/qhc.12.3.194
Runciman WB, Roughead EE, Semple SJ, Adams RJ (2003) Adverse drug events and medication errors in Australia. Int J Qual Health Care 15(90001):i49–i59. https://doi.org/10.1093/intqhc/mzg085
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM (2004) Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 329(7456):15–19. https://doi.org/10.1136/bmj.329.7456.15
Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R et al (1995) Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE prevention study group. JAMA 274(1):29–34. https://doi.org/10.1001/jama.1995.03530010043033
Kohn LT, Corrigan JM, Donaldson MS (2000) Errors in health care: a leading cause of death and injury
Willadsen TG, Bebe A, Køster-Rasmussen R, Jarbøl DE, Guassora AD, Waldorff FB, Reventlow S, Olivarius NF (2016) The role of diseases, risk factors and symptoms in the definition of multimorbidity—a systematic review. Scand J Prim Health Care 34(2):112–121. https://doi.org/10.3109/02813432.2016.1153242
Nexøe J, Halvorsen PA, Kristiansen IS (2007) Review article: critiques of the risk concept—valid or not? Scand J Public Health 35(6):648–654. https://doi.org/10.1080/14034940701418897
Reventlow S, Hvas AC, Tulinius C (2001) In Really great danger?. The concept of risk in general practice. Scand J Prim Health Care 19(2):71–75
Giorda CB, Nada E, Tartaglino B, Marafetti L, Gnavi RA (2014) Systematic review of acute pancreatitis as an adverse event of type 2 diabetes drugs: from hard facts to a balanced position. Diabetes Obes Metab 16(11):1041–1047. https://doi.org/10.1111/dom.12297
Aronson JK (2013) Distinguishing hazards and harms, adverse drug effects and adverse drug reactions: implications for drug development, clinical trials, pharmacovigilance, biomarkers, and monitoring. Drug Saf 36(3):147–153. https://doi.org/10.1007/s40264-013-0019-9
Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP (1997) Adverse drug events in hospitalized patients: excess length of stay, extra costs, and attributable mortality. JAMA 277(4):301–306. https://doi.org/10.1001/jama.1997.03540280039031
Porter J, Jick H (1977) Drug-related deaths among medical inpatients. JAMA 237(9):879–881. https://doi.org/10.1001/jama.1977.03270360041015
Hug BL, Keohane C, Seger DL, Yoon C, Bates DW (2012) The costs of adverse drug events in community hospitals. Jt Comm J Qual Patient Saf 38(3):120–126. https://doi.org/10.1016/S1553-7250(12)38016-1
Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW (2003) The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 138(3):161–167. https://doi.org/10.7326/0003-4819-138-3-200302040-00007
Ernst FR, Grizzle AJ (2001) Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (1996) 41(2):192–199. https://doi.org/10.1016/S1086-5802(16)31229-3
Rudd RA, Aleshire N, Zibbell JE, Matthew Gladden R (2016) Increases in drug and opioid overdose deaths—United States, 2000–2014. Am J Transplant 16(4):1323–1327. https://doi.org/10.1111/ajt.13776
Alomar MJ (2014) Factors affecting the development of adverse drug reactions (review article). Saudi Pharm J 22(2):83–94. https://doi.org/10.1016/j.jsps.2013.02.003
Kiguba R, Karamagi C, Bird SM (2017) Incidence, risk factors and risk prediction of hospital-acquired suspected adverse drug reactions: a prospective cohort of Ugandan inpatients. BMJ Open 7(1):e010568. https://doi.org/10.1136/bmjopen-2015-010568
Boeker EB, Ram K, Klopotowska JE, Boer M, Creus MT, Andrés AL, Sakuma M, Morimoto T, Boermeester MA, Dijkgraaf MGW (2015) An individual patient data meta-analysis on factors associated with adverse drug events in surgical and non-surgical inpatients. Br J Clin Pharmacol 79(4):548–557. https://doi.org/10.1111/bcp.12504
Al Hamid A, Ghaleb M, Aljadhey H, Aslanpour ZA (2014) Systematic review of hospitalization resulting from medicine-related problems in adult patients. Br J Clin Pharmacol 78(2):202–217. https://doi.org/10.1111/bcp.12293
Resende LSO, Santos-Neto ET (2015) Risk factors associated with adverse reactions to antituberculosis drugs. J Bras Pneumol 41(1):77–89. https://doi.org/10.1590/S1806-37132015000100010
Stelzer G, Dalah I, Stein TI, Satanower Y, Rosen N, Nativ N, Oz-Levi D, Olender T, Belinky F, Bahir I, Krug H, Perco P, Mayer B, Kolker E, Safran M, Lancet D (2011) In-silico human genomics with GeneCards. Hum Genomics 5(6):709–717. https://doi.org/10.1186/1479-7364-5-6-709
Piatetsky-Shapiro G (1991) Discovery, analysis, and presentation of strong rules. In: Piatetsky-Shapiro G, Frawley WJ (eds) Knowledge discovery in databases. AAAI/MIT Press, Cambridge
Hahsler M, Grün B, Hornik K (2005) arules-A computational environment for mining association rules and frequent item sets. J Stat Softw 14(15):1–25. https://doi.org/10.18637/jss.v014.i15
Härkänen M, Kervinen M, Ahonen J, Voutilainen A, Turunen H, Vehviläinen-Julkunen K (2015) Patient-specific risk factors of adverse drug events in adult inpatients–evidence detected using the global trigger tool method. J Clin Nurs 24(3-4):582–591. https://doi.org/10.1111/jocn.12714
Mugoša S (2015) Adverse drug reactions in hospitalised cardiac patients: risk factors. Vojnosanit Pregl 72(11):975–981. https://doi.org/10.2298/VSP140710104M
Van den Bemt P, Egberts ACG, Lenderink AW, Verzijl JM, Simons KA, Van der Pol W, Leufkens HGM (2000) Risk factors for the development of adverse drug events in hospitalized patients. Pharm World Sci 22(2):62–66. https://doi.org/10.1023/A:1008721321016
Pedrós C, Quintana B, Rebolledo M, Porta N, Vallano A, Arnau JM (2014) Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur J Clin Pharmacol 70(3):361–367. https://doi.org/10.1007/s00228-013-1630-5
Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R, Gambassi G (2002) Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc 50(12):1962–1968. https://doi.org/10.1046/j.1532-5415.2002.50607.x
Friedman BW, Cisewski DH, Holden L, Bijur PE, Gallagher EJ (2015) Age but not sex is associated with efficacy and adverse events following administration of intravenous migraine medication: an analysis of a clinical trial database. Headache: J Head Face Pain 55(10):1342–1355. https://doi.org/10.1111/head.12697
Zopf Y, Rabe C, Neubert A, Gassmann KG, Rascher W, Hahn EG, Brune K, Dormann H (2008) Women encounter ADRs more often than do men. Eur J Clin Pharmacol 64(10):999–1004. https://doi.org/10.1007/s00228-008-0494-6
Bradbury F (2004) How important is the role of the physician in the correct use of a drug? An observational cohort study in general practice. Int J Clin Pract 58:27–32
Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, Fukui T, Bates DW (2004) An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 10(4):499–509. https://doi.org/10.1111/j.1365-2753.2003.00484.x
Minkowitz HS, Gruschkus SK, Shah M, Raju A (2014) Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm 71(18):1556–1565. https://doi.org/10.2146/ajhp130031
Macedo AF, Alves C, Craveiro N, Marques FB (2011) Multiple drug exposure as a risk factor for the seriousness of adverse drug reactions. J Nurs Manag 19(3):395–399. https://doi.org/10.1111/j.1365-2834.2011.01216.x
Shiozawa T, Tadokoro J-i, Fujiki T, Fujino K, Kakihata K, Masatani S, Morita S, Gemma A, Boku N (2013) Risk factors for severe adverse effects and treatment-related deaths in Japanese patients treated with irinotecan-based chemotherapy: a postmarketing survey. Jpn J Clin Oncol 43(5):483–491. https://doi.org/10.1093/jjco/hyt040
de Boer M, Boeker EB, Ramrattan MA, Kiewiet JJS, Dijkgraaf MGW, Boermeester MA, Lie-A-Huen L (2013) Adverse drug events in surgical patients: an observational multicentre study. Int J Clin Pharm 35(5):744–752. https://doi.org/10.1007/s11096-013-9797-5
Kongkaew C, Hann M, Mandal J, Williams SD, Metcalfe D, Noyce PR, Ashcroft DM (2013) Risk factors for hospital admissions associated with adverse drug events. Pharmacotherapy: J Hum Pharmacol Drug Ther 33(8):827–837. https://doi.org/10.1002/phar.1287
Lee JS, Yang J, Stockl KM, Lew H, Solow BK (2016) Evaluation of eligibility criteria used to identify patients for medication therapy management services: a retrospective cohort study in a Medicare advantage part D population. J Manag Care Specialty Pharm 22(1):22–30. 10.18553/jmcp.2016.22.1.22
Field TS, Gurwitz JH, Harrold LR, Rothschild J, DeBellis KR, Seger AC, Auger JC, Garber LA, Cadoret C, Fish LS (2004) Risk factors for adverse drug events among older adults in the ambulatory setting. J Am Geriatr Soc 52(8):1349–1354. https://doi.org/10.1111/j.1532-5415.2004.52367.x
Helldén A, Bergman U, von Euler M, Hentschke M, Odar-Cederlöf I, Öhlén G (2009) Adverse drug reactions and impaired renal function in elderly patients admitted to the emergency department. Drugs Aging 26(7):595–606. https://doi.org/10.2165/11315790-000000000-00000
Zhang B, Dong Y, Liang L, Lian Z, Liu J, Luo X, Chen W, Li X, Liang C, Zhang S (2016) The incidence, classification, and management of acute adverse reactions to the low-osmolar iodinated contrast media Isovue and Ultravist in contrast-enhanced computed tomography scanning. Medicine 95(12):e3170. https://doi.org/10.1097/MD.0000000000003170
Gülbay BE, Gürkan ÖU, Yıldız ÖA, Önen ZP, Erkekol FÖ, Baççıoğlu A, Acıcan T (2006) Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med 100(10):1834–1842. https://doi.org/10.1016/j.rmed.2006.01.014
Admassie E, Melese T, Mequanent W, Hailu W, Srikanth BA (2013) Extent of poly-pharmacy, occurrence and associated factors of drug-drug interaction and potential adverse drug reactions in Gondar Teaching Referral Hospital, North West Ethiopia. J Adv Pharm Technol Res 4(4):183–189. https://doi.org/10.4103/2231-4040.121412
Rashed AN, Wong ICK, Cranswick N, Tomlin S, Rascher W, Neubert A (2012) Risk factors associated with adverse drug reactions in hospitalised children: international multicentre study. Eur J Clin Pharmacol 68(5):801–810. https://doi.org/10.1007/s00228-011-1183-4
Buchbinder R, Forbes A, Kobben F, Boyd I, Snow RM, McNeil JJ (2000) Clinical features of tiaprofenic acid (surgam) associated cystitis and a study of risk factors for its development. J Clin Epidemiol 53(10):1013–1019. https://doi.org/10.1016/S0895-4356(00)00192-X
Geer MI, Koul PA, Tanki SA, Shah MY (2016) Frequency, types, severity, preventability and costs of adverse drug reactions at a tertiary care hospital. J Pharmacol Toxicol Methods 81:323–334. https://doi.org/10.1016/j.vascn.2016.04.011
Thiesen S, Conroy EJ, Bellis JR, Bracken LE, Mannix HL, Bird KA, Duncan JC, Cresswell L, Kirkham JJ, Peak M (2013) Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children—sa prospective observational cohort study of 6,601 admissions. BMC Med 11:1
Hartikainen S, Mäntyselkä P, Louhivuori-Laako K, Enlund H, Sulkava R (2005) Concomitant use of analgesics and psychotropics in home-dwelling elderly people-Kuopio 75+ study. Br J Clin Pharmacol 60(3):306–310. https://doi.org/10.1111/j.1365-2125.2005.02417.x
Bellis JR, Kirkham JJ, Thiesen S, Conroy EJ, Bracken LE, Mannix HL, Bird KA, Duncan JC, Peak M, Turner MA (2013) Adverse drug reactions and off-label and unlicensed medicines in children: a nested case? Control study of inpatients in a pediatric hospital. BMC Med 11:1
Sheth HS, Verrico MM, Skledar SJ, Towers AL (2005) Promethazine adverse events after implementation of a medication shortage interchange. Ann Pharmacother 39(2):255–261. https://doi.org/10.1345/aph.1E361
Chang H-M, Tsai H-C, Lee SS-J, Kunin C, Lin P-C, Wann S-R, Chen Y-S (2016) High daily doses of trimethoprim/sulfamethoxazole are an independent risk factor for adverse reactions in patients with pneumocystis pneumonia and AIDS. J Chin Med Assoc 79(6):314–319. https://doi.org/10.1016/j.jcma.2016.01.007
Kim KS, Moon A, Kang HJ, Shin HY, Choi YH, Kim HS, Kim SG (2016) Higher plasma bilirubin predicts veno-occlusive disease in early childhood undergoing hematopoietic stem cell transplantation with cyclosporine. World J Transplant 6(2):403–410. https://doi.org/10.5500/wjt.v6.i2.403
Prows CA, Zhang X, Huth MM, Zhang K, Saldaña SN, Daraiseh NM, Esslinger HR, Freeman E, Greinwald JH, Martin LJ (2014) Codeine-related adverse drug reactions in children following tonsillectomy: a prospective study. Laryngoscope 124(5):1242–1250. https://doi.org/10.1002/lary.24455
Langerová P, Vrtal J, Urbánek K (2014) Adverse drug reactions causing hospital admissions in childhood: a prospective, observational, single-centre study. Basic Clin Pharmacol Toxicol 115(6):560–564. https://doi.org/10.1111/bcpt.12264
Singh H, Kumar BN, Sinha T, Dulhani N (2011) The incidence and nature of drug-related hospital admission: a 6-month observational study in a tertiary health care hospital. J Pharmacol Pharmacother 2(1):17–20. https://doi.org/10.4103/0976-500X.77095
Rodenburg EM, Stricker BH, Visser LE (2012) Sex differences in cardiovascular drug-induced adverse reactions causing hospital admissions. Br J Clin Pharmacol 74(6):1045–1052. https://doi.org/10.1111/j.1365-2125.2012.04310.x
Harugeri A, Parthasarathi G, Ramesh M, Guido S, Basavanagowdappa H (2011) Frequency and nature of adverse drug reactions in elderly in-patients of two Indian medical college hospitals. J Postgrad Med 57(3):189–195. https://doi.org/10.4103/0022-3859.85201
Montastruc JL, Lapeyre-Mestre M, Bagheri H, Fooladi A (2002) Gender differences in adverse drug reactions: analysis of spontaneous reports to a regional Pharmacovigilance Centre in France. Fundam Clin Pharmacol 16(5):343–346. https://doi.org/10.1046/j.1472-8206.2002.00100.x
Schwaab B, Katalinic A, Böge UM, Loh J, Blank P, Kölzow T, Poppe D, Bonnemeier H (2009) Quinidine for pharmacological cardioversion of atrial fibrillation: a retrospective analysis in 501 consecutive patients. Ann Noninvasive Electrocardiol 14(2):128–136. https://doi.org/10.1111/j.1542-474X.2009.00287.x
Zopf Y, Rabe C, Neubert A, Hahn EG, Dormann H (2008) Risk factors associated with adverse drug reactions following hospital admission. Drug Saf 31(9):789–798. https://doi.org/10.2165/00002018-200831090-00007
Bravo AER, Tchambaz L, Krähenbühl-Melcher A, Hess L, Schlienger RG, Krähenbühl S (2005) Prevalence of potentially severe drug-drug interactions in ambulatory patients with dyslipidaemia receiving HMG-CoA reductase inhibitor therapy. Drug Saf 28(3):263–275. https://doi.org/10.2165/00002018-200528030-00007
Gervasoni C, Meraviglia P, Landonio S, Baldelli S, Fucile S, Castagnoli L, Clementi E, Riva A, Galli M, Rizzardini G (2013) Low body weight in females is a risk factor for increased tenofovir exposure and drug-related adverse events. PLoS One 8(12):e80242. https://doi.org/10.1371/journal.pone.0080242
Ahmad N, Javaid A, Syed Sulaiman SA, Afridi AK, Zainab, Khan AH (2016) Occurrence, management, and risk factors for adverse drug reactions in multidrug resistant tuberculosis patients. Am J Ther. https://doi.org/10.1097/MJT.0000000000000421
Field TS, Gurwitz JH, Avorn J, McCormick D, Jain S, Eckler M, Benser M, Bates DW (2001) Risk factors for adverse drug events among nursing home residents. Arch Intern Med 161(13):1629–1634. https://doi.org/10.1001/archinte.161.13.1629
Shi W, Wang Y-M, Li S-L, Yan M, Li D, Chen B-Y, Cheng N-N (2004) Risk factors of adverse drug reaction from non-steroidal anti-inflammatory drugs in shanghai patients with arthropathy. Acta Pharmacol Sin 25(3):357–365
Chrischilles E, Rubenstein L, Van Gilder R, Voelker M, Wright K, Wallace R (2007) Risk factors for adverse drug events in older adults with mobility limitations in the community setting. J Am Geriatr Soc 55(1):29–34. https://doi.org/10.1111/j.1532-5415.2006.01034.x
Fiszenson-Albala F, Auzerie V, Mahe E, Farinotti R, Durand-Stocco C, Crickx B, Descamps V (2003) A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol 149(5):1018–1022. https://doi.org/10.1111/j.1365-2133.2003.05584.x
Shah R, Gajjar B, Desai S (2012) A profile of adverse drug reactions with risk factors among geriatric patients in a tertiary care teaching rural hospital in India. Natl J Physiol Pharm Pharmacol 2(2):113–122. https://doi.org/10.5455/njppp.2012.2.113-122.
Shinde KM, Pore SM, Bapat TR (2013) Adverse reactions to first-line anti-tuberculous agents in hospitalised patients: pattern, causality, severity and risk factors. Indian J Med Specialities 4(1):16–21
Bertulyte I, Schwan S, Hallberg P (2014) Identification of risk factors for carbamazepine-induced serious mucocutaneous adverse reactions: a case-control study using data from spontaneous adverse drug reaction reports. J Pharmacol Pharmacother 5(2):100–138. https://doi.org/10.4103/0976-500X.130051
Abera W, Cheneke W, Abebe G (2016) Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis patients in Dawro Zone, South Ethiopia: a cohort study. Int J Mycobacteriol 5(1):14–20. https://doi.org/10.1016/j.ijmyco.2015.10.002
Li H, Shi Q (2015) Drugs and diseases interacting with cigarette smoking in US prescription drug labelling. Clin Pharmacokinet 54(5):493–501. https://doi.org/10.1007/s40262-015-0246-6
Chrischilles EA, VanGilder R, Wright K, Kelly M, Wallace RB (2009) Inappropriate medication use as a risk factor for self-reported adverse drug effects in older adults. J Am Geriatr Soc 57(6):1000–1006. https://doi.org/10.1111/j.1532-5415.2009.02269.x
Lattanzio F, Laino I, Pedone C, Corica F, Maltese G, Salerno G, Garasto S, Corsonello A, Incalzi RA (2012) PharmacosurVeillance in the elderly care study G. Geriatric conditions and adverse drug reactions in elderly hospitalized patients. J Am Med Dir Assoc 13(2):96–99. https://doi.org/10.1016/j.jamda.2011.04.006
Haile DB, Ayen WY, Tiwari P (2013) Prevalence and assessment of factors contributing to adverse drug reactions in wards of a tertiary care hospital, India. Ethiop J Health Sci 23(1):39–48
Green JL, Hawley JN, Rask KJI (2007) The number of prescribing physicians an independent risk factor for adverse drug events in an elderly outpatient population? Am J Geriatr Pharmacother 5(1):31–39. https://doi.org/10.1016/j.amjopharm.2007.03.004
Lu CY, Roughead E (2011) Determinants of patient-reported medication errors: a comparison among seven countries. Int J Clin Pract 65:733–740
Tuchinda P, Chularojanamontri L, Sukakul T, Thanomkitti K, Nitayavardhana S, Jongjarearnprasert K, Uthaitas P, Kulthanan K (2014) Cutaneous adverse drug reactions in the elderly: a retrospective analysis in Thailand. Drugs Aging 31(11):815–824. https://doi.org/10.1007/s40266-014-0209-x
Lopes LC, Silveira MS, de Camargo MC, de Camargo IA, Luz TCB, Osorio-de-Castro CG, Barberato-Filho S, del Fiol Fde S, Guyatt G (2014) Patient reports of the frequency and severity of adverse reactions associated with biological agents prescribed for psoriasis in Brazil. Expert Opin Drug Saf 13(9):1155–1163. https://doi.org/10.1517/14740338.2014.942219
De Smedt RHE, Denig P, van der Meer K, Haaijer-Ruskamp FM, Jaarsma T (2011) Self-reported adverse drug events and the role of illness perception and medication beliefs in ambulatory heart failure patients: a cross-sectional survey. Int J Nurs Stud 48(12):1540–1550. https://doi.org/10.1016/j.ijnurstu.2011.05.014
Hung S-I, Chung W-H, Liou L-B, Chu C-C, Lin M, Huang H-P, Lin Y-L, Lan J-L, Yang L-C, Hong H-S (2005) HLA-B* 5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A 102(11):4134–4139. https://doi.org/10.1073/pnas.0409500102
Corsonello A, Pedone C, Corica F, Mazzei B, Di Iorio A, Carbonin P, Incalzi RA (2005) Concealed renal failure and adverse drug reactions in older patients with type 2 diabetes mellitus. J Gerontol Ser A Biol Med Sci 60(9):1147–1151. https://doi.org/10.1093/gerona/60.9.1147
Komeda T, Ishii S, Itoh Y, Sanekata M, Yoshikawa T, Shimada J (2016) Post-marketing safety evaluation of the intravenous anti-influenza neuraminidase inhibitor peramivir: a drug-use investigation in patients with high risk factors. J Infect Chemother 22(10):677–684. https://doi.org/10.1016/j.jiac.2016.07.004
Chen Y-C, Fan J-S, Chen M-H, Hsu T-F, Huang H-H, Cheng K-W, Yen DH-T, Huang C-I, Chen L-K, Yang C-C (2014) Risk factors associated with adverse drug events among older adults in emergency department. Eur J Intern Med 25(1):49–55. https://doi.org/10.1016/j.ejim.2013.10.006
Mok S, Minson Q (2008) Drug-related problems in hospitalized patients with HIV infection. Am J Health Syst Pharm 65(1):55–59. https://doi.org/10.2146/ajhp070011
Namazi S, Pourhatami S, Borhani-Haghighi A, Roosta S (2014) Incidence of potential drug-drug interaction and related factors in hospitalized neurological patients in two Iranian teaching hospitals. Iran J Med Sci 39(6):515–521
Patel TK, Bhabhor PH, Desai N, Shah S, Patel PB, Vatsala E, Panigrahi S (2015) Adverse drug reactions in a psychiatric department of tertiary care teaching hospital in India: analysis of spontaneously reported cases. Asian J Psychiatr 17:42–49. https://doi.org/10.1016/j.ajp.2015.07.003
Wawruch M, Zikavska M, Wsolova L, Kuzelova M, Kahayova K, Strateny K, Kristova V (2009) Adverse drug reactions related to hospital admission in Slovak elderly patients. Arch Gerontol Geriatr 48(2):186–190. https://doi.org/10.1016/j.archger.2008.01.004
Bejhed RS, Kharazmi M, Hallberg P (2016) Identification of risk factors for bisphosphonate-associated atypical femoral fractures and osteonecrosis of the jaw in a pharmacovigilance database. Ann Pharmacother 50(8):616–624. https://doi.org/10.1177/1060028016649368
Onder G, Penninx BWJH, Landi F, Atkinson H, Cesari M, Bernabei R, Pahor M (2003) Depression and adverse drug reactions among hospitalized older adults. Arch Intern Med 163(3):301–305. https://doi.org/10.1001/archinte.163.3.301
Masip M, Tuneu L, Pagès N, Torras X, Gallego A, Guardiola JM, Faus MJ, Mangues MA (2015) Prevalence and detection of neuropsychiatric adverse effects during hepatitis C treatment. Int J Clin Pharm 37(6):1143–1151. https://doi.org/10.1007/s11096-015-0177-1
Rashed AN, Wilton L, Lo CCH, Kwong B, Leung S, Wong ICK (2014) Epidemiology and potential risk factors of drug-related problems in Hong Kong paediatric wards. Br J Clin Pharmacol 77(5):873–879. https://doi.org/10.1111/bcp.12270
Indradat S, Veskitkul J, Pacharn P, Jirapongsananuruk O, Visitsunthorn N (2016) Provocation proven drug allergy in Thai children with adverse drug reactions. Asian Pac J Allergy Immunol 34(1):59–64. 10.12932/AP0601.34.1.2016
Liao PJ, Shih CP, Mao CT, Deng ST, Hsieh MC, Hsu KH (2013) The cutaneous adverse drug reactions: risk factors, prognosis and economic impacts. Int J Clin Pract 67(6):576–584. https://doi.org/10.1111/ijcp.12097
Fosnes GS, Lydersen S, Farup PG (2011) Constipation and diarrhoea-common adverse drug reactions? A cross sectional study in the general population. BMC Pharmacol Toxicol 11:2
Salvi F, Rossi L, Lattanzio F, Cherubini A (2016) Is Polypharmacy an independent risk factor for adverse outcomes after an emergency department visit?. Intern Emerg Med 12(2):213–220. https://doi.org/10.1007/s11739-016-1451-5
Roulet L, Ballereau F, Hardouin J-B, Chiffoleau A, Potel G, Asseray N (2014) Adverse drug event nonrecognition in emergency departments: an exploratory study on factors related to patients and drugs. J Emerg Med 46(6):857–864. https://doi.org/10.1016/j.jemermed.2013.11.124
Thuermann PA, Windecker R, Steffen J, Schaefer M, Tenter U, Reese E, Menger H, Schmitt K (2002) Detection of adverse drug reactions in a neurological department. Drug Saf 25(10):713–724. https://doi.org/10.2165/00002018-200225100-00004
Varallo FR, Capucho HC, Planeta CS, Mastroianni PC (2011) Safety assessment of potentially inappropriate medications (PIM) use in older people and the factors associated with hospital admission. J Pharm Pharm Sci 14(2):283–290. 10.18433/J3P01J
Varallo FR, Capucho HC, da Silva Planeta C, de Carvalho Mastroianni P (2014) Possible adverse drug events leading to hospital admission in a Brazilian teaching hospital. Clinics 69(3):163–167. https://doi.org/10.6061/clinics/2014(03)03
Saiyed MM, Lalwani T, Rana DI (2015) Off-label use a risk factor for adverse drug reactions in pediatric patients? A prospective study in an Indian tertiary care hospital. Int J Risk Saf Med 27:45–53
de Araújo Lobo MGA, Pinheiro SMB, Castro JGD, Momenté VG, Pranchevicius M-CS (2013) Adverse drug reaction monitoring: support for pharmacovigilance at a tertiary care hospital in Northern Brazil. BMC Pharmacol Toxicol 14:1
Tangiisuran B, Davies JG, Wright JE, Rajkumar C (2012) Adverse drug reactions in a population of hospitalized very elderly patients. Drugs Aging 29(8):669–679. https://doi.org/10.2165/11632630-000000000-00000
Admassie E, Melese T, Mequanent W, Hailu W, Srikanth BA (2013) Extent of poly-pharmacy, occurrence and associated factors of drug-drug interaction and potential adverse drug reactions in Gondar Teaching Referral Hospital, North West Ethiopia. J Adv Pharm Technol Res 4:183
Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc J-L, Lapeyre-Mestre M (2009) Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department. Drugs Aging 26(6):475–482. https://doi.org/10.2165/00002512-200926060-00004
Smithburger PL, Buckley MS, Culver MA, Sokol S, Lat I, Handler SM, Kirisci L, Kane-Gill SLA (2015) Multicenter evaluation of off-label medication use and associated adverse drug reactions in adult medical ICUs. Crit Care Med 43(8):1612–1621. https://doi.org/10.1097/CCM.0000000000001022
Zandieh SO, Goldmann DA, Keohane CA, Yoon C, Bates DW, Kaushal R (2008) Risk factors in preventable adverse drug events in pediatric outpatients. J Pediatr 152(2):225–231. https://doi.org/10.1016/j.jpeds.2007.09.054
Trivalle C, Burlaud A, Ducimetière P, Group I (2011) Risk factors for adverse drug events in hospitalized elderly patients: a geriatric score. Eur Geriatr Med 2(5):284–289. https://doi.org/10.1016/j.eurger.2011.07.002
Zyoud SH, Awang R, Sulaiman S, Azhar S, Al-Jabi SW (2011) N-acetylcysteine-induced headache in hospitalized patients with acute acetaminophen overdose. Fundam Clin Pharmacol 25(3):405–410. https://doi.org/10.1111/j.1472-8206.2010.00831.x
Botelho LFF, Porro AM, Enokihara MMSS, Tomimori J (2015) Adverse cutaneous drug reactions in a single quaternary referral hospital. Int J Dermatol 55(4):e198–e203. https://doi.org/10.1111/ijd.13126
Singh PK, Kumar MK, Kumar D, Kumar P (2015) Morphological pattern of cutaneous adverse drug reactions due to antiepileptic drugs in eastern India. J Clin Diagn Res: JCDR 9(1):WC01–WC03. https://doi.org/10.7860/JCDR/2015/11701.5419
Yang CY, Dao RL, Lee TJ, Lu CW, Yang CH, Hung SI, Chung WH (2011) Severe cutaneous adverse reactions to antiepileptic drugs in Asians. Neurology 77(23):2025–2033. https://doi.org/10.1212/WNL.0b013e31823b478c
Anwikar SR, Bandekar MS, Smrati B, Pazare AP, Tatke PA, Kshirsagar NA (2011) HAART induced adverse drug reactions: a retrospective analysis at a tertiary referral health care center in India. Int J Risk Saf Med 23:163–169
Castelán-Martínez OD, Jiménez-Méndez R, Rodríguez-Islas F, Fierro-Evans M, Vázquez-Gómez BE, Medina-Sansón A, Clark P, Carleton B, Ross C, Hildebrand C (2014) Hearing loss in Mexican children treated with cisplatin. Int J Pediatr Otorhinolaryngol 78(9):1456–1460. https://doi.org/10.1016/j.ijporl.2014.06.007
Passarelli MCG, Jacob-Filho W, Figueras A (2005) Adverse drug reactions in an elderly hospitalised population. Drugs Aging 22(9):767–777. https://doi.org/10.2165/00002512-200522090-00005
Shalviri G, Yousefian S, Gholami K (2012) Adverse events induced by ceftriaxone: a 10-year review of reported cases to Iranian Pharmacovigilance Centre. J Clin Pharm Ther 37(4):448–451. https://doi.org/10.1111/j.1365-2710.2011.01321.x
Sachs B, Fischer-Barth W, Erdmann S, Merk HF, Seebeck J (2007) Anaphylaxis and toxic epidermal necrolysis or Stevens–Johnson syndrome after nonmucosal topical drug application: fact or fiction? Allergy 62(8):877–883. https://doi.org/10.1111/j.1398-9995.2007.01398.x
Jayarama N, Shiju K, Prabahakar K (2012) Adverse drug reactions in adults leading to emergency department visits. Int J Pharm Pharm Sci 4:642–646
Nguyen DV, Chu HC, Nguyen DV, Phan MH, Craig T, Baumgart K, van Nunen S (2015) HLA-B* 1502 and carbamazepine-induced severe cutaneous adverse drug reactions in Vietnamese. Asia Pac Allergy 5(2):68–77. https://doi.org/10.5415/apallergy.2015.5.2.68
Jiang J, Tang Q, Feng J, Dai R, Wang Y, Yang Y, Tang X, Deng C, Zeng H, Zhao Y (2016) Association between SLCO1B1− 521T> C and− 388A> G polymorphisms and risk of statin-induced adverse drug reactions: A meta-analysis. SpringerPlus 5(1):1368. https://doi.org/10.1186/s40064-016-2912-z
Sun D, Yu C-H, Liu Z-S, He X-L, Hu J-S, Wu G-F, Mao B, Wu S-H, Xiang H-H (2014) Association of HLA-B* 1502 and* 1511 allele with antiepileptic drug-induced Stevens-Johnson syndrome in central China. J Huazhong Univ Sci Technol Med Sci 34:146–150
Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe’er I, Floratos A, Daly MJ, Goldstein DB, John S, Nelson MR (2009) HLA-B* 5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet 41(7):816–819. https://doi.org/10.1038/ng.379
Kaniwa N, Saito Y (2013) The risk of cutaneous adverse reactions among patients with the HLA-A* 31: 01 allele who are given carbamazepine, oxcarbazepine or eslicarbazepine: a perspective review. Ther Adv Drug Saf 4(6):246–253. https://doi.org/10.1177/2042098613499791
Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, Ikezawa Z, Iijima M, Shiohara T, Hashimoto K (2011) Genome-wide association study identifies HLA-A* 3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet 20(5):1034–1041. https://doi.org/10.1093/hmg/ddq537
Kim S-H, Jee Y-K, Lee J-H, Lee B-H, Kim Y-S, Park J-S, Kim S-H (2012) ABCC2 haplotype is associated with antituberculosis drug-induced maculopapular eruption. Allergy, Asthma Immunol Res 4(6):362–366. https://doi.org/10.4168/aair.2012.4.6.362
Yi JH, Cho Y-J, Kim W-J, Lee MG, Lee JH (2013) Genetic variations of ABCC2 gene associated with adverse drug reactions to valproic acid in Korean epileptic patients. Genom Inform 11(4):254–262. https://doi.org/10.5808/GI.2013.11.4.254
Visscher H, Ross CJD, Rassekh SR, Barhdadi A, Dubé M-P, Al-Saloos H, Sandor GS, Caron HN, van Dalen EC, Kremer LC, Canadian Pharmacogenomics Network for Drug Safety, C (2011) Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol 30(13):1422–1428. https://doi.org/10.1200/jco.2010.34.3467
Saruwatari J, Matsunaga M, Ikeda K, Nakao M, Oniki K, Seo T, Mihara S, Marubayashi T, Kamataki T, Nakagawa K (2006) Impact of CYP2D6* 10 on H1-antihistamine-induced hypersomnia. Eur J Clin Pharmacol 62(12):995–1001. https://doi.org/10.1007/s00228-006-0210-3
Lee A-Y, Kim M-J, Chey W-Y, Choi J, Kim B-G (2004) Genetic polymorphism of cytochrome P450 2C9 in diphenylhydantoin-induced cutaneous adverse drug reactions. Eur J Clin Pharmacol 60(3):155–159. https://doi.org/10.1007/s00228-004-0753-0
Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N, Whittaker P, Ranganath V, Kumanduri V, McLaren W, Holm L, Lindh J, Rane A, Wadelius M, Deloukas PA (2009) Genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet 5(3):e1000433. https://doi.org/10.1371/journal.pgen.1000433
Pare G, Kubo M, Byrd JB, McCarty CA, Woodard-Grice A, Teo KK, Anand SS, Zuvich RL, Bradford Y, Ross S (2013) Genetic variants associated with angiotensin-converting enzyme inhibitor-associated angioedema. Pharmacogenet Genomics 23(9):470–478. https://doi.org/10.1097/FPC.0b013e328363c137
Sadhasivam S, Zhang X, Chidambaran V, Mavi J, Pilipenko V, Mersha TB, Meller J, Kaufman KM, Martin LJ, McAuliffe J (2015) Novel associations between FAAH genetic variants and postoperative central opioid-related adverse effects. Pharmacogenomics J 15(5):436–442. https://doi.org/10.1038/tpj.2014.79
Misra UK, Kalita J, Nair PP (2010) Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J Neurol Sci 293(1-2):12–17. https://doi.org/10.1016/j.jns.2010.03.025
Kalow W (1961) Unusual responses to drugs in some hereditary conditions. Can Anaesth Soc J 8:43–52
Manolio TA (2010) Genomewide association studies and assessment of the risk of disease. N Engl J Med 363(2):166–176. https://doi.org/10.1056/NEJMra0905980
Denham MJ (1990) Adverse drug reactions. Br Med Bull 46(1):53–62. https://doi.org/10.1093/oxfordjournals.bmb.a072394
Batchelor JR, Welsh KI, Tinoco RM, Dollery CT, Hughes GR, Bernstein R, Ryan P, Naish PF, Aber GM, Bing RF, Russell GI (1980) Hydralazine-induced systemic lupus erythematosus: influence of HLA-DR and sex on susceptibility. Lancet (London, England) 1:1107–1109
Teplitz L, Igic R, Berbaum ML, Schwertz DW (2005) Sex differences in susceptibility to epinephrine-induced arrhythmias. J Cardiovasc Pharmacol 46(4):548–555. https://doi.org/10.1097/01.fjc.0000179435.26373.81
Rees JL, Friedmann PS, Matthews JNS (1989) Sex differences in susceptibility to development of contact hypersensitivity to dinitrochlorobenzene (DNCB). Br J Dermatol 120(3):371–374. https://doi.org/10.1111/j.1365-2133.1989.tb04162.x
Kotseva K, Wood D, De Backer G, De Bacquer D, Pyörälä K, Keil U, Group ES. EUROASPIRE III (2009) A survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European countries. Eur J Cardiovasc Prev Rehabil 16(2):121–137. https://doi.org/10.1097/HJR.0b013e3283294b1d
Fortescue EB, Kaushal R, Landrigan CP, McKenna KJ, Clapp MD, Federico F, Goldmann DA, Bates DW (2003) Prioritizing strategies for preventing medication errors and adverse drug events in pediatric inpatients. Pediatrics 111(4):722–729. https://doi.org/10.1542/peds.111.4.722
Hanlon JT, Schmader KE, Koronkowski MJ, Weinberger M, Landsman PB, Samsa GP, Lewis IK (1997) Adverse drug events in high risk older outpatients. J Am Geriatr Soc 45(8):945–948. https://doi.org/10.1111/j.1532-5415.1997.tb02964.x
Acknowledgements
The authors would like to thank one reviewer for her assistance in annotating a subset of research articles. The authors would also like to thank another reviewer who provided consultation for several subtypes of risk factors.
Author information
Authors and Affiliations
Contributions
LZ: data annotation and categorization; idea conceiving and study design; analysis and interpretation of the data; and preparation, review, revisions, and approval of the manuscript. AR: collection, management, annotation, analysis, and interpretation of the data; and preparation, revisions, and approval of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 196 kb)
Rights and permissions
About this article
Cite this article
Zhou, L., Rupa, A.P. Categorization and association analysis of risk factors for adverse drug events. Eur J Clin Pharmacol 74, 389–404 (2018). https://doi.org/10.1007/s00228-017-2373-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2373-5