Abstract
Purpose
The aim of this clinical study was to investigate a previously proposed mechanism of ketoconazole-mediated inhibition of cytochrome P450 3A (CYP3A) induction.
Methods
A two-phase, randomized, cross-over, open, mono-centre trial was carried out. Participants received ketoconazole and St John’s wort for 8 days to study the proposed suppression of St John’s wort-mediated induction of CYP3A at the transcriptional level. In the second phase, we studied the inhibitory effect of a single dose of ketoconazole directly at the enzyme level during CYP3A induction by St John’s wort. Midazolam served as a marker substance of CYP3A activity using an established limited sampling strategy.
Results
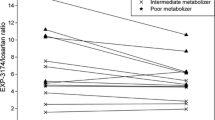
After 8 days of simultaneous ketoconazole and St John’s wort administration, CYP3A-mediated midazolam metabolism was strongly inhibited (81 % decrease in clearance). Following the induction of CYP3A with St John’s wort (6.6-fold increase in clearance on day 8), a single dose of ketoconazole strongly inhibited midazolam metabolism to the same degree (82 % decrease in clearance in relation to baseline). An induction of midazolam metabolism was observed after discontinuation of both drugs in both study phases. These results apparently contradict the in vitro results where ketoconazole showed an inhibitory effect on the transcription of CYP3A genes.
Conclusions
Ketoconazole is a strong inhibitor of CYP3A, also when used concomitantly with St John’s wort. In therapeutic doses it does not inhibit pregnane X receptor-mediated induction of CYP3A in vivo.



Similar content being viewed by others
References
Guengerich FP (1999) Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 39:1–17
Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA (2000) St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA 97(13):7500–7502
Chen J, Raymond K (2006) Roles of rifampicin in drug-drug interactions: underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann Clin Microbiol Antimicrob 5:3
Huang H, Wang H, Sinz M, Zoeckler M, Staudinger J, Redinbo MR, Teotico DG, Locker J, Kalpana GV, Mani S (2007) Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene 26(2):258–268
Wang H, Huang H, Li H, Teotico DG, Sinz M, Baker SD, Staudinger J, Kalpana G, Redinbo MR, Mani S (2007) Activated pregnenolone X-receptor is a target for ketoconazole and its analogs. Clin Cancer Res 13(8):2488–2495
Chen Y, Tang Y, Robbins GT, Nie D (2010) Camptothecin attenuates cytochrome P450 3A4 induction by blocking the activation of human pregnane X receptor. J Pharmacol Exp Ther 334(3):999–1008
Healan-Greenberg C, Waring JF, Kempf DJ, Blomme EA, Tirona RG, Kim RB (2008) A human immunodeficiency virus protease inhibitor is a novel functional inhibitor of human pregnane X receptor. Drug Metab Dispos 36(3):500–507
Ekins S, Kholodovych V, Ai N, Sinz M, Gal J, Gera L, Welsh WJ, Bachmann K, Mani S (2008) Computational discovery of novel low micromolar human pregnane X receptor antagonists. Mol Pharmacol 74(3):662–672
Hafner V, Jager M, Matthee AK, Ding R, Burhenne J, Haefeli WE, Mikus G (2010) Effect of simultaneous induction and inhibition of CYP3A by St John’s Wort and ritonavir on CYP3A activity. Clin Pharmacol Ther 87(2):191–196
Katzenmaier S, Markert C, Mikus G (2010) Proposal of a new limited sampling strategy to predict CYP3A activity using a partial AUC of midazolam. Eur J Clin Pharmacol 66(11):1137–1141
Katzenmaier S, Markert C, Riedel KD, Burhenne J, Haefeli WE, Mikus G (2011) Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using A limited sampling strategy. Clin Pharmacol Ther 90(5):666–673
Quintela O, Cruz A, Concheiro M, de Castro A, Lopez-Rivadulla M (2004) A sensitive, rapid and specific determination of midazolam in human plasma and saliva by liquid chromatography/electrospray mass spectrometry. Rapid Commun Mass Spectrom 18(24):2976–2982
US Department of Health and Human Services FDA (2001) Guidance for industry, bioanalytical method validation. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28(5):481–504
Dresser GK, Schwarz UI, Wilkinson GR, Kim RB (2003) Coordinate induction of both cytochrome P4503A and MDR1 by St John’s wort in healthy subjects. Clin Pharmacol Ther 73(1):41–50
Xie R, Tan LH, Polasek EC, Hong C, Teillol-Foo M, Gordi T, Sharma A, Nickens DJ, Arakawa T, Knuth DW, Antal EJ (2005) CYP3A and P-glycoprotein activity induction with St. John’s Wort in healthy volunteers from 6 ethnic populations. J Clin Pharmacol 45(3):352–356
Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CY (2002) Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther 72(3):276–287
Chen M, Nafziger AN, Bertino JS Jr (2006) Drug-metabolizing enzyme inhibition by ketoconazole does not reduce interindividual variability of CYP3A activity as measured by oral midazolam. Drug Metab Dispos 34(12):2079–2082
Lee JI, Chaves-Gnecco D, Amico JA, Kroboth PD, Wilson JW, Frye RF (2002) Application of semisimultaneous midazolam administration for hepatic and intestinal cytochrome P450 3A phenotyping. Clin Pharmacol Ther 72(6):718–728
Krishna G, Moton A, Ma L, Savant I, Martinho M, Seiberling M, McLeod J (2009) Effects of oral posaconazole on the pharmacokinetic properties of oral and intravenous midazolam: a phase I, randomized, open-label, crossover study in healthy volunteers. Clin Ther 31(2):286–298
Bohmer GM, Drollmann A, Gleiter CH, Nave R (2008) Effect of coadministered ketoconazole, a strong cytochrome P450 3A4 enzyme inhibitor, on the pharmacokinetics of ciclesonide and its active metabolite desisobutyryl-ciclesonide. Clin Pharmacokinet 47(5):343–349
Svecova L, Vrzal R, Burysek L, Anzenbacherova E, Cerveny L, Grim J, Trejtnar F, Kunes J, Pour M, Staud F, Anzenbacher P, Dvorak Z, Pavek P (2008) Azole antimycotics differentially affect rifampicin-induced pregnane X receptor-mediated CYP3A4 gene expression. Drug Metab Dispos 36(2):339–348
Stoch SA, Friedman E, Maes A, Yee K, Xu Y, Larson P, Fitzgerald M, Chodakewitz J, Wagner JA (2009) Effect of different durations of ketoconazole dosing on the single-dose pharmacokinetics of midazolam: shortening the paradigm. J Clin Pharmacol 49(4):398–406
Olkkola KT, Backman JT, Neuvonen PJ (1994) Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther 55(5):481–485
Daneshmend TK, Warnock DW, Ene MD, Johnson EM, Potten MR, Richardson MD, Williamson PJ (1984) Influence of food on the pharmacokinetics of ketoconazole. Antimicrob Agents Chemother 25(1):1–3
Garg V, Chandorkar G, Yang Y, Adda N, McNair L, Alves K, Smith F, van Heeswijk RP (2012) The effect of CYP3A inhibitors and inducers on the pharmacokinetics of telaprevir in healthy volunteers. Br J Clin Pharmacol. doi: 10.1111/j.1365-2125.2012.04345.x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuchs, I., Hafner-Blumenstiel, V., Markert, C. et al. Effect of the CYP3A inhibitor ketoconazole on the PXR-mediated induction of CYP3A activity. Eur J Clin Pharmacol 69, 507–513 (2013). https://doi.org/10.1007/s00228-012-1388-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1388-1