Abstract
Purpose
To determine the influence of itraconazole on the pharmacokinetics, and the CNS and prolactin-elevating effects of domperidone in humans.
Methods
Fifteen healthy volunteers received either itraconazole (200 mg daily) or placebo for 5 days with a double blind, randomized, cross-over design. A single oral 20-mg dose of domperidone was administered to subjects on day 5. Plasma domperidone and serum prolactin concentrations were measured. The effects of domperidone on CNS were also assessed using self-rating scales and electroencephalography.
Results
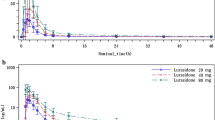
Itraconazole significantly increased domperidone AUC0-∞ (3.2-fold) and Cmax (2.7-fold) compared with placebo, but had no significant effect on the elimination half-life of domperidone. The CNS effects of domperidone assessed by self-rating of mood and electroencephalography, and the prolactin-elevating effect, were not significantly affected by itraconazole. A counterclockwise hysteresis was evident in the relationship between plasma domperidone and serum prolactin concentrations. Itraconazole shifted the hysteresis to the right. Concentration–effect modeling procedures yielded a significant linear relationship between hypothetical effect site domperidone concentrations and prolactin levels. Itraconazole reduced the slope of the linear relationship.
Conclusions
Itraconazole significantly increased plasma domperidone concentrations. The interaction is probably mainly due to a reduced first pass elimination by inhibition of CYP3A and/or MDR1. The clinical significance of the altered relationship between domperidone concentrations and prolactin levels caused by itraconazole is still to be determined.



Similar content being viewed by others
References
Reddymasu SC, Soykan I, McCallum RW (2007) Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol 102:2036–2045
Masaoka T, Tack J (2009) Gastroparesis: current concepts and management. Gut Liver 3:166–173
Heykants J, Hendriks R, Meuldermans W, Michiels M, Scheygrond H, Reyntjens H (1981) On the pharmacokinetics of domperidone in animals and man. IV. The pharmacokinetics of intravenous domperidone and its bioavailability in man following intramuscular, oral and rectal administration. Eur J Drug Metab Pharmacokinet 6:61–70
Ward BA, Morocho A, Kandil A, Galinsky RE, Flockhart DA, Desta Z (2004) Characterization of human cytochrome P450 enzymes catalyzing domperidone N-dealkylation and hydroxylation in vitro. Br J Clin Pharmacol 58:277–287
El Ela AA, Härtter S, Schmitt U et al (2004) Identification of P-glycoprotein substrates and inhibitors among psychoactive compounds-implications for pharmacokinetics of selected substrates. J Pharm Pharmacol 56:967–975
Schinkel AH, Wagenaar E, Mol CA, van Deemter L (1996) P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 97:2517–2524
Dan Y, Murakami H, Koyabu N, Ohtani H, Sawada Y (2002) Distribution of domperidone into the rat brain is increased by brain ischaemia or treatment with the P-glycoprotein inhibitor verapamil. J Pharm Pharmacol 54:729–733
Patterson D, Abell T, Rothstein R, Koch K, Barnett J (1999) A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol 94:1230–1234
Tsujikawa K, Dan Y, Nogawa K, Sato H, Yamada Y, Murakami H, Ohtani H, Sawada Y, Iga T (2003) Potentiation of domperidone-induced catalepsy by a P-glycoprotein inhibitor, cyclosporin A. Biopharm Drug Dispos 24:105–114
Wang E-J, Lew K, Casciano CN, Clement RP, Johnson WW (2002) Interaction of common azole antifungals with P-glycoprotein. Antimicrob Agents Chemother 46:160–165
Pauli-Magnus C, von Richter O, Burk O, Ziegler A, Mettang T, Eichelbaum M, Fromm MF (2000) Characterization of the major metabolites of verapamil as substrates and inhibitors of P-glycoprotein. J Pharmacol Exp Ther 293:376–382
Hughes J, Crowe A (2010) Inhibition of P-glycoprotein-mediated efflux of digoxin and its metabolites by macrolide antibiotics. J Pharmacol Sci 113:315–324
Backman JT, Kivistö KT, Olkkola KT, Neuvonen PJ (1998) The area under the plasma concentration-time curve for oral midazolam is 400-fold larger during treatment with itraconazole than with rifampicin. Eur J Clin Pharmacol 54:53–58
Varhe A, Olkkola KT, Neuvonen PJ (1994) Oral triazolam is potentially hazardous to patients receiving systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther 56:601–607
Shon J-H, Yoon Y-R, Hong W-S, Nguyen P-M, Lee S-S, Choi Y-G, Cha I-J, Shin J-G (2005) Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin Pharmacol Ther 78:191–201
Ohtani Y, Kotegawa T, Tsutsumi K, Morimoto T, Hirose Y, Nakano S (2002) Effect of fluconazole on the pharmacokinetics and pharmacodynamics of oral and rectal bromazepam: an application of electroencephalography as the pharmacodynamic method. J Clin Pharmacol 42:183–191
Inada T, Beasley CM, Tanaka Y, Walker DJ (2003) Extrapyramidal symptom profiles assessed with the Drug-Induced Extrapyramidal Symptom Scale: comparison with Western scales in the clinical double-blind studies of schizophrenic patients treated with either olanzapine or haloperidol. Int Clin Psychopharmacol 18:39–48
Imanaga J, Kotegawa T, Imai H, Tsutsumi K, Yoshizato T, Ohyama T, Shirasaka Y, Tamai I, Tateishi T, Kyoichi O (2011) The effects of the SLCO2B1 c.1457C>T polymorphism and apple juice on the pharmacokinetics of fexofenadine and midazolam in humans. Pharmacogenet Genomics 21:84–93
Holford NH, Sheiner LB (1981) Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet 6:429–453
Greenblatt DJ, Harmatz JS (1993) Kinetic-dynamic modeling in clinical psychopharmacology. J Clin Psychopharmacol 13:231–234
Damkier P, Hansen LL, Brosen K (1999) Effect of diclofenac, disulfiram, itraconazole, grapefruit juice and erythromycin on the pharmacokinetics of quinidine. Br J Clin Pharmacol 48:829–838
Faassen F, Vogel G, Spanings H, Vromans H (2003) Caco-2 permeability, P-glycoprotein transport ratios and brain penetration of heterocyclic drugs. Int J Pharm 263:113–122
Jalava KM, Partanen J, Neuvonen PJ (1997) Itraconazole decreases renal clearance of digoxin. Ther Drug Monit 19:609–613
Brouwers JRBJ, Assies J, Wiersinga WM, Huizingn G, Tytgat GN (1980) Plasma prolactin levels after acute and subchronic oral administration of domperidone and of metoclopramide: a cross-over study in healthy volunteers. Clin Endocrinol 12:435–440
Nerozzi D, Magnani A, Sforza V, Scaramucci E, Cerilli M, Moretti C, Frajese G, Antonozzi I, Meltzer HY (1990) Plasma prolactin response to domperidone in acute schizophrenia and schizophreniform illness. Psychiatry Res 34:139–147
Schwartz JB, Verotta D, Sheiner LB (1989) Pharmacodynamic modeling of verapamil effects under steady-state and nonsteady-state conditions. J Pharmacol Exp Ther 251:1032–1038
Meredith PA, Kelman AW, Elliott HL, Reid JL (1983) Pharmacokinetic and pharmacodynamic modeling of trimazosin and its major metabolite. J Pharmacokinet Biopharm 11:323–335
Nashan D, Knuth UA, Weidinger G, Nieschlag E (1989) The antimycotic drug terbinafine in contrast to ketoconazole lacks acute effects on the pituitary-testicular function of healthy men: a placebo-controlled double-blind trial. Acta Endocrinol 120:677–681
Bateman DN, Kahn C, Mashiter K, Davies DS (1978) Pharmacokinetic and concentration-effect studies with intravenous metoclopramide. Br J Clin Pharmacol 6:401–407
Coppola R, Herrmann WM (1987) Psychotropic drug profiles: comparisons by topographic maps of absolute power. Neuropsychobiology 18:97–104
Lee DY, Lee KU, Kwon JS, Jang IJ, Cho MJ, Shin SG, Woo JI (1999) Pharmacokinetic-pharmacodynamic modeling of risperidone effects on electroencephalography in healthy volunteers. Psychopharmacology 144:272–278
Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, Weimar W, van Gelder T (2003) Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther 74:245–254
Renders L, Frisman M, Ufer M, Mosyagin I, Haenisch S, Ott U, Caliebe A, Dechant M, Braun F, Kunzendorf U, Cascorbi I (2007) CYP3A5 genotype markedly influences the pharmacokinetics of tacrolimus and sirolimus in kidney transplant recipients. Clin Pharmacol Ther 81:228–234
Micaud V, Simard C, Turgeon J (2010) Characterization of CYP3A isozymes involves in the metabolism of domperidone: role of cytochrome b(5) and inhibition by ketoconazole. Drug Metab Lett 4:95–103
Kharasch ED, Walker A, Isoherranen N, Hoffer C, Sheffels P, Thummel K, Whittington D, Ensign D (2007) Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther 82:410–426
Fromm MF, Schwilden H, Bachmakov I, Köning J, Bremer F, Schüttler J (2007) Impact of the CYP3A5 genotype on midazolam pharmacokinetics and pharmacodynamics during intensive care sedation. Eur J Clin Pharmacol 63:1129–1133
Acknowledgements
This study was supported in part by a grant from Japan Research Foundation for Clinical Pharmacology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshizato, T., Kotegawa, T., Imai, H. et al. Itraconazole and domperidone: a placebo-controlled drug interaction study. Eur J Clin Pharmacol 68, 1287–1294 (2012). https://doi.org/10.1007/s00228-012-1258-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1258-x