Abstract
Purpose
Knowledge of the pharmacokinetics (PK) of plasma-derived factor IX (FIX) is still inadequate, with conflicting findings on its elimination half-life and as yet no analysis of the variance in PK between and within individuals. The aim of the study was thus to characterize the PK of plasma-derived FIX, including estimates of variance between and within patients, in adult patients and to predict the variation between individuals in dose requirement to produce a target trough level during regular prophylaxis.
Methods
Plasma FIX versus time data were compiled from four published and one unpublished PK study involving a total of 26 adult patients with severe haemophilia B. The number of PK assessments per patient varied between one and eight, yielding in total 893 measured FIX levels from 80 study occasions. A population PK model was developed to describe the whole dataset. Parameter values from the model were used to calculate the dose requirement to maintain a trough level of 1% of normal FIX activity in each patient.
Results
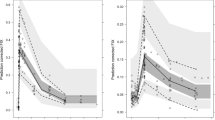
The disposition of FIX was well described by a three-compartment PK model. The median elimination half-life was 31 h, and the variation between individuals was modest both in PK and in dose requirement during twice-weekly prophylaxis.
Conclusion
With twice weekly dosing, the need for PK-based dose tailoring of FIX in adult patients appears to be limited. However, monitoring FIX levels should be considered in children, in patients who do not respond satisfactorily to standard dosing, and if treatment is switched from plasma-derived to recombinant FIX.



Similar content being viewed by others
References
Escobar MA (2003) Treatment on demand—in vivo dose finding studies. Haemophilia 9:360–367
World Federation of Hemophilia (2008) Protocols for the treatment of hemophilia and von Willebrand disease, 3rd edn. Treatment of Hemophilia monograph no 14. Available at: http://www.wfh.org/2/docs/Publications Accessed 16 June 2010.
Nilsson IM, Berntorp E, Löfqvist T, Pettersson H (1992) Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Int Med 232:25–32
Björkman S (2003) Prophylactic dosing of factor VIII and factor IX from a clinical pharmacokinetic perspective. Haemophilia 9[Suppl 1]:101–110
Collins PW, Fischer K, Morfini M, Blanchette VS, Björkman S (2011) Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia 17:2–10
Björkman S, Carlsson M (1997) The pharmacokinetics of factor VIII and factor IX: methodology, pitfalls and applications. Haemophilia 3:1–8
Björkman S, Berntorp E (2001) Pharmacokinetics of coagulation factors: clinical relevance for patients with haemophilia. Clin Pharmacokinet 40:815–832
Björkman S (2011) A commentary on the differences in pharmacokinetics between recombinant and plasma-derived factor IX and their implications for dosing. Haemophilia 17:179–184
Björkman S, Carlsson M, Berntorp E (1994) Pharmacokinetics of factor IX in patients with haemophilia B: methodological aspects and physiological interpretation. Eur J Clin Pharmacol 46:325–332
The European Agency for the Evaluation of Medicinal Products (EMEA), Committee for Proprietary Medicinal Products (CPMP) (2000). Note for guidance on the clinical investigation of human plasma derived factor VIII and IX products (CPMP/BPWG/198/95 rev. 1). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003621.pdf. Accessed 12 July 2011
Hoag MS, Johnson FF, Robinson JA, Aggeler PM (1969) Treatment of hemophilia B with a new clotting factor concentrate. New Engl J Med 280:581–586
Berntorp E, Björkman S, Carlsson M, Lethagen S, Nilsson IM (1993) Biochemical and in vivo properties of high purity factor IX concentrates. Thrombos Haemostas 70:768–773
White GC, Beebe A, Nielsen B (1997) Recombinant factor IX. Thromb Haemost 78:261–265
Goudemand J, Peynet J, Chambost H, Négrier C, Briquel ME, Claeyssens S, Derlon-Borel A, Guérois C, Caron C, Scherrmann JM, Debray M, Bridey F (1998) A cross-over pharmacokinetic study of a double viral inactivated factor IX concentrate (15 nm filtration and SD) compared to a SD factor IX concentrate. Thromb Haemost 80:919–924
Aznar JA, Cabrera N, Matysiak M, Zawilska K, Gercheva L, Antonov A, Montañés M, Páez AM, Lissitchkov T (2009) Pharmacokinetic study of a high-purity factor IX concentrate (Factor IX Grifols) with a 6-month follow up in previously treated patients with severe haemophilia B. Haemophilia 15:1243–1248
Lissitchkov T, Matysiak M, Zawilska K, Łaguna P, Gercheva L, Antonov A, Cabrera N, Aznar JA, Woodward MK, Páez A (2011) A clinical study assessing the pharmacokinetics, efficacy and safety of AlphaNine, a high-purity factor IX concentrate, in patients with severe haemophilia B. Haemophilia 17:590–596
Serban M, Skotnicki AB, Colovic M, Jinca C, Klukowska A, Laguna P, Wolf DM (2011) Clinical efficacy, safety and pharmacokinetic properties of the plasma-derived factor IX concentrate Haemonine in previously treated patients with severe haemophilia B. Haemophilia 17. doi:10.1111/j.1365-2516.2011.02624.x
Fijnvandraat K, Berntorp E, ten Cate JW, Johnsson H, Peters M, Savidge G, Tengborn L, Spira J, Stahl C (1997) Recombinant, B-domain deleted factor VIII (r-VIII SQ): Pharmacokinetics and initial safety aspects in hemophilia A patients. Thromb Haemost 77:298–302
Björkman S, Folkesson A, Jönsson S (2009) Pharmacokinetics and dose requirements of factor VIII over the age range 3-74 years: A population analysis based on 50 patients with long-term prophylactic treatment for haemophilia A. Eur J Clin Pharmacol 65:989–998
Björkman S, Blanchette VS, Fischer K, Oh M, Spotts G, Schroth P, Fritsch S, Patrone L, Ewenstein BW, for the ADVATE Clinical Program Group, Collins PW (2010) Comparative pharmacokinetics of plasma- and albumin-free recombinant factor VIII in children and adults: the influence of blood sampling schedule on observed age-related differences and implications for dose tailoring. J Thrombos Haemostas 8:730–736
Carlsson M, Björkman S, Berntorp E (1998) Multidose pharmacokinetics of factor IX: implications for dosing in prophylaxis. Haemophilia 4:83–88
Björkman S, Shapiro AD, Berntorp E (2001) Pharmacokinetics of recombinant factor IX in relation to age of the patient: implications for dosing in prophylaxis. Haemophilia 7:133–139
Ahnström J, Berntorp E, Lindvall K, Björkman S (2004) A six-year follow-up of dosing, coagulation factor levels and bleedings in relation to joint status in the prophylactic treatment of haemophilia. Haemophilia 10:689–697
Giddings JC (1980) Hereditary coagulation disorders: laboratory techniques. In: Thomson JM (ed) Blood coagulation and haemostasis. Churchill Livingstone, New York, pp 117–157
Karlsson MO, Sheiner LB (1993) The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 21:735–750
Jonsson EN, Karlsson MO (1999) Xpose—an S-PLUS based population pharmacokinetic-pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64
Meibohm B, Läer S, Panetta JC, Barrett JS (2005) Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J 7:475–787
Thomson AH, Whiting B (1992) Bayesian parameter estimation and population pharmacokinetics. Clin Pharmacokin 22:447–467
Upton RN (2004) Calculating the hybrid (macro) rate constants of a three-compartment mammillary pharmacokinetic model from known micro-rate constants. J Pharmacol Toxicol Method 49:65–68
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82:17–20
Savic RM, Karlsson MO (2009) Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J 11:558–569
Karlsson MO, Holford N. A tutorial on visual predictive checks. PAGE 17 (2008) Abstract 1434. Available at: http://www.page-meeting.org/default.asp?abstract=1434. Accessed 12 July 2011
Stonebraker JS, Bolton-Maggs PHB, Soucie JM, Walkers I, Brooker M (2011) A study of variations in the reported haemophilia B prevalence around the world. Haemophilia 17. doi:10.1111/j.1365-2516.2011.02588.x
Chitlur M, Warrier I, Rajpurkar M, Lusher JM (2009) Inhibitors in factor IX deficiency a report of the ISTH-SSC international FIX inhibitor registry (1997-2006). Haemophilia 15:1027–1031
Holford NHG (1996) A size standard for pharmacokinetics. Clin Pharmacokinet 30:329–332
Collins PW, Björkman S, Fischer K, Blanchette V, Oh M, Schroth P, Fritsch S, Casey K, Spotts G, Ewenstein BW (2010) Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe haemophilia A: influences of variance in pharmacokinetics and treatment regimens. J Thrombos Haemostas 8:269–275
Schimpf K, Baumann P (1976) Die ambulante Dauerbehandling der Hämophilie B; eine kontrollierte Studie (A controlled study of long-term treatment of haemophilia B on an out-patient basis). Dtsch Med Wochenschr 101:233–238
Björkman S (2010) Limited blood sampling for pharmacokinetic dose tailoring of FVIII in the prophylactic treatment of haemophilia A. Haemophilia 16:597–605
Acknowledgements
The authors wish to thank Bio Products Laboratory for making these data available, Rukhsana Shaikh-Zaidi for providing additional information whenever needed and Peter Collins for helping us to find data and to get in touch with the company, and for critical reading of the manuscript. The original researchers in the Replenine-VF clinical study were Dr. P. Collins (Cardiff), Dr. C. Raper (Hull), Dr. V. Mitchell (Leicester), Dr. D. Prangnell (Lincoln), Dr. T. Nokes (Basingstoke), Professor C.A. Lee (London) and Dr. T. Baglin (Cambridge). The authors also wish to thank Anna Folkesson, Siv Jönsson, Akash Khandelwal and Kajsa Harling for advice and technical help in various stages of the modeling.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Björkman, S., Åhlén, V. Population pharmacokinetics of plasma-derived factor IX in adult patients with haemophilia B: implications for dosing in prophylaxis. Eur J Clin Pharmacol 68, 969–977 (2012). https://doi.org/10.1007/s00228-012-1211-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1211-z