Abstract
Background
Prophylaxis with factor VIII (FVIII) should be individualized based on patient characteristics, including FVIII pharmacokinetics. Population pharmacokinetic (popPK) modeling simplifies pharmacokinetic studies by obviating the need for multiple samples.
Objective
The objective of this study was to characterize the pharmacokinetics and inter-individual variability (IIV) of BAY 94-9027 in relation to patient characteristics in support of a popPK-tailored approach, including identifying the optimal number and timing of pharmacokinetic samples.
Methods
Pharmacokinetic samples from 198 males (aged 2‒62 years) with severe hemophilia A, enrolled in BAY 94-9027 clinical trials, were analyzed. Baseline age, height, weight, body mass index, lean body weight (LBW), von Willebrand factor (VWF) level, and race were evaluated. A popPK model was developed and used to simulate pharmacokinetic endpoints difficult to observe from measured FVIII levels, including time to maintain FVIII levels above 1, 3, and 5 IU/dL after different BAY 94-9027 doses.
Results
A one-compartment model adequately described BAY 94-9027 pharmacokinetics. Clearance and central volume of distribution were significantly associated with LBW; clearance was inversely correlated with VWF. Due to the monophasic pharmacokinetics and well-understood IIV sources, identification of patient pharmacokinetics was achievable with sparse blood sampling. Median predicted time to maintain FVIII levels > 1 IU/dL in patients aged ≥ 12 years ranged from 120.1 to 127.2 h after single BAY 94-9027 doses of 45‒60 IU/kg.
Conclusions
This analysis evaluated the pharmacokinetics of BAY 94-9027 and its sources of IIV. Using the model, determination of individual patient pharmacokinetics was possible with few FVIII samples, and a sparse sampling design to support pharmacokinetic-guided dosing was identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A robust population pharmacokinetics (popPK) model was derived for BAY 94-9027 using data from three BAY 94-9027 clinical trials, including 198 patients with severe hemophilia A. |
BAY 94-9027 pharmacokinetics were adequately described using a one-compartment model in which lean body weight explained much of the inter-patient variability seen in clearance and volume of distribution of the central compartment. |
The popPK model can be used to accurately determine BAY 94-9027 pharmacokinetic profiles for individual patients using sparse blood sampling. |
1 Introduction
Hemophilia A is a congenital bleeding disorder resulting from reduced levels of coagulation factor VIII (FVIII) [1]. Patients with severe hemophilia A, FVIII level < 1 IU/dL, are especially at risk for spontaneous bleeding episodes, often into muscles and joints [1].
The current standard of care for severe hemophilia A is prophylaxis with intravenous infusions with FVIII to prevent bleeding episodes [2]. Differences among patients in FVIII pharmacokinetic profile can influence the prophylactic doses required to maintain optimal FVIII levels; among other pharmacokinetic properties, the FVIII half-life influences time to maintain FVIII levels above a critical threshold [3]. Thus, an individualized approach that considers the patient’s FVIII pharmacokinetic characteristics, as well as age, bleeding frequency, and level of physical activity [1, 4], could be used to determine prophylaxis regimens that achieve FVIII levels above a desired threshold and reduce the risk of underdosing [3, 5]. The Scientific and Standardization Committee (SSC) of the International Society on Thrombosis and Haemostasis (ISTH) has issued a rationale for adoption of a population pharmacokinetic (popPK) approach to individualization of hemophilia treatment and practical guidance on implementing popPKs in clinical practice [6, 7].
BAY 94-9027 is an extended-half-life, B-domain-deleted (BDD) recombinant FVIII (rFVIII), site-specifically conjugated with 60 kDa branched polyethylene glycol (PEG) at a cysteine, introduced into the A3 domain (K1804C), resulting in one PEG per BDD–rFVIII protein [8]. Efficacy and safety of BAY 94-9027 for on-demand treatment of bleeding episodes and prophylaxis in previously treated patients with severe hemophilia A were evaluated in phase I and II/III trials [8,9,10]. It is approved for prophylaxis and on-demand treatment of bleeding episodes in patients with hemophilia A in the USA [11], Canada, Japan, and the European Union.
This work aimed to develop a popPK model to characterize pharmacokinetics of BAY 94-9027, particularly inter-individual variability (IIV) in pharmacokinetics and its relationship to patient characteristics. Using this popPK model, we identified the minimum number of FVIII samples required and the optimal sampling time to support pharmacokinetic-guided dosing. This model intends to use simulations to quantify pharmacokinetic endpoints of interest that are not otherwise observed directly from measured FVIII levels, such as time to reach FVIII levels of 1, 3, and 5 IU/dL after BAY 94-9027 infusion.
2 Methods
2.1 Included Studies
The popPK analysis included pharmacokinetic data from patients enrolled in three BAY 94-9027 clinical trials (a phase I study [NCT01184820] [8], PROTECT VIII [NCT01580293] [9], and PROTECT VIII Kids [NCT01775618] [10]). Patients were aged 21–58 years (phase I study, n = 14), 12–62 years (PROTECT VIII, n = 132), or 2–11 years (PROTECT VIII Kids, n = 60) with severe hemophilia A (FVIII < 1 IU/dL). All patients had been previously treated with a FVIII product other than BAY 94-9027 (≥ 150 exposure days in the phase I study and PROTECT VIII; > 50 exposure days in PROTECT VIII Kids). Patients with a history, or current evidence, of FVIII inhibitors were excluded from the trials. Each study was performed according to study protocol and was approved by the independent ethics committee/institutional review board at participating sites.
The popPK model was developed during the clinical development program of BAY 94-9027 comprising all available pharmacokinetic data across studies. However, as the approval was granted for patients aged ≥ 12 years only, the inferences from this analysis are limited to this population.
In the phase I study, a dense pharmacokinetic sampling design was used (preinfusion and 11 samples up to 168 h postinfusion). In PROTECT VIII, sparse sampling (preinfusion and one sample 15 min postinfusion) was used for all patients, and dense sampling (preinfusion and ten samples up to 96 h postinfusion) was used for a pharmacokinetic subgroup. For PROTECT VIII Kids, sparse samples (recovery and/or preinfusion) were obtained in all patients; dense sampling (preinfusion and five samples up to 72 h postinfusion) was used for the pharmacokinetic subgroup. In the phase I and PROTECT VIII studies, samples were assessed using one-stage and chromogenic assays; in PROTECT VIII Kids, the chromogenic assay was used. The lower limit of quantification (LLOQ) was 1.5–3.0 IU/dL for the chromogenic assay and 1.0–6 IU/dL for the one-stage assay. The one-stage assay used HemosIL® SynthAFax (Instrumentation Laboratory, Bedford, MA, USA) phospholipid reagent containing ellagic acid, standard human plasma (Siemens, Malvern, PA, USA), and the ACL Advance System (Instrumentation Laboratory). The chromogenic assay used BIOPHEN™ FVIII:C chromogenic assay kit (HYPHEN BioMed, Neuville-sur-Oise, France) with product standard in microplate format on the Sunrise™ absorbance microplate reader (Tecan, Männedorf, Switzerland).
Data from 206 male patients aged 2–62 years, including 60 patients aged < 12 years, who had either full pharmacokinetic profiles (n = 65) or sparse samples (n = 141), assessed using a chromogenic assay, were used for model development. Pharmacokinetic data below the LLOQ (BLQ) were accounted for in the analysis; 28 samples from seven children who either developed anti-PEG antibodies, had perceived loss of efficacy, or both, and from one patient in PROTECT VIII with a drug hypersensitivity reaction were excluded. These eight patients withdrew from the study and had insufficient pharmacokinetic and recovery data.
2.2 BAY 94-9027 Population Pharmacokinetic Modeling
The popPK analysis was conducted by means of non-linear mixed-effects modeling using the software NONMEM® version 7.2 (ICON Development Solutions, Ellicott City, MD, USA). A non-linear mixed-effects model consists of fixed- and random-effects components; while fixed effects describe the structural model and covariate relationship, which are constant across subjects, the random effects components describe the observed variability. In the final model structure, to account for BLQ samples, the likelihood-based methods for imputation of these values (M3 method) was applied to avoid bias in half-life estimates [5].
To describe the residual unexplained variability (RUV), a so-called combined error model consisting of a concentration-proportional and a concentration-independent additive part was used. If the pre-specified error model turned out to be mis-specified, different error models, such as proportional or additive, could be tested.
Potential change in pharmacokinetics over time was explored by assessing inter-occasion variability (IOV). By means of the final model structure and covariate selection, a model fit was determined using one-stage assay data, available for 1648 samples from 132 and 14 subjects in the PROTECT VIII and phase I studies, respectively.
2.3 Covariate Modeling
After qualification of the base model, the influence of covariates on pharmacokinetic parameters was tested for statistical significance. The parameters of interest for the covariate analysis were clearance (CL) and volume of distribution of the central compartment (Vc). Covariates assessed were baseline age, height, weight, body mass index, lean body weight (LBW), von Willebrand factor (VWF) antigen level, and race.
As no VWF measurements were available from the PROTECT VIII Kids study, the influence of VWF on BAY 94-9027 pharmacokinetics was only informed by adult/adolescent data. Different scenarios were evaluated, including imputation of missing values in PROTECT VIII Kids of the median VWF from the phase I and PROTECT VIII studies, and assessment of VWF effect on patients in the phase I and PROTECT VIII studies only. The latter was conducted to assess whether the imputation based on adult/adolescent data introduces bias into the analysis.
Covariates were selected using stepwise forward/backward selection [12]. First, covariates were screened for their relevance using a univariate statistical test; covariates were candidates for the final popPK model if inclusion in the model resulted in a decrease in objective function value (ΔOFV) of ≥ 6.63 (p < 0.01). All candidate covariates were combined into a full model. The relevance of each covariate in a multivariate context was tested by removing each covariate from the full model; only covariates for which removal led to an increase in ΔOFV of ≥ 7.88 (p < 0.005) were included in the final model.
Differences between chromogenic and one-stage assays were evaluated by Bland–Altman assessment, where mean FVIII activity between assays were compared.
2.4 Model Evaluation
The popPK model was qualified using standard diagnostic tools, including goodness-of-fit plots, standard errors of parameter estimates, and shrinkage estimates. The primary method of model validation was the visual predictive check (VPC) [13]. Before VPC statistics were calculated, BLQ values were assigned a value of 0.5 × LLOQ of the respective sample.
2.5 Evaluation of Sparse Sampling Design Strategies
The dataset for this analysis consisted of all available densely sampled profiles (57 in total) from 36 patients > 12 years of age with a median of 11 postinfusion samples (range 7‒12). Samples were collected between 0.5 and 168 h postinfusion from patients who received either 25 or 60 IU/kg BAY 94-9027. Dense profiles were artificially reduced to sparse profiles of three samples (collected at 4, 24, and 48 h; Björkman design [14]), two samples (collected at 4 and 48 h; reduced Björkman design), or one sample (all sampling times tested individually). The final popPK model (including covariates) was applied to the artificially reduced sparse profiles to derive individual Bayesian post hoc estimates for CL and Vc.
For 21 patients, two dense profiles were obtained. These profiles were used to evaluate the influence of potential IOV on the robustness of the most promising one-sample sampling strategy.
2.6 Model Simulations
To evaluate whether prophylactic dosing with BAY 94-9027 could maintain sufficient FVIII levels, data from the final popPK model were used to perform simulations to estimate time to reach FVIII levels of 1, 3, and 5 IU/dL after single 25–60 IU/kg doses of BAY 94-9027 and to estimate the percentage of patients treated once weekly, twice weekly, or every 5 days with trough FVIII levels above thresholds of 1, 3, or 5 IU/dL at steady state. The specific regimens evaluated, with increments of 5 IU/kg within each dose range, were 60 IU/kg once weekly, 25–40 IU/kg twice weekly, and 45–60 IU/kg every 5 days. Simulations are shown for patients aged ≥ 12 years.
3 Results
3.1 Patients and Pharmacokinetic Dataset
Baseline continuous covariates were available for all 198 patients, including 53 patients aged < 12 years, and were included in the final model (Table 1). For the categorical covariate of race, 144 of the 198 patients (72.7%) were white, 35 (17.7%) were Asian, 9 (4.5%) were black, and one (0.5%) was Native American; race was not reported for nine patients (4.5%).
The final model was fitted using 2196 pharmacokinetic samples (455 BLQ samples [21%]), and BLQ observations increased over time postdose (Fig. 1). An evaluation of the impact of assay used included an additional 1648 samples (257 BLQ samples [16%]) from the one-stage assay analysis.
3.2 Development of Base Model for BAY 94-9027
BAY 94-9027 pharmacokinetic data were adequately described by a one-compartment model with first-order elimination (Fig. 2). While a two-compartment model was considered, the peripheral compartment typically describing an early distribution phase for FVIII kinetics could not be identified, indicated by poor estimates of the intercompartmental CL (relative standard error > 70%). Additionally, the goodness-of-fit plots did not show any trend for structural model mis-specification using a one-compartment model.
CL and Vc were strongly associated with all size-related covariates, and thus this relationship was included in the stability base model. LBW appeared to have the strongest relationship with CL and Vc, resulting in the largest OFV decrease (ΔOFV − 509.92), and was chosen as the most appropriate size-related covariate. Both CL and Vc were non-linearly related to LBW, with an estimated exponent of 0.610 (95% confidence interval [CI] 0.466–0.754) for CL and 0.872 (95% CI 0.816–0.928) for Vc. Considering the effect of LBW on both CL and Vc removed the trend in empirical Bayesian estimate versus age, weight, and height, inclusion of additional size-related covariates was not pursued. Including LBW decreased unexplained IIV, expressed as percentage coefficient of variation (CV), from 47.5 to 32.7% for CL and from 43.2 to 12.7% for Vc.
No consistent change in CL or Vc over time at a population level was seen when IOV was applied to all data (Fig. 3). IOV for both CL and Vc could be estimated with moderate precision (CV < 50%). The magnitude of IOV was considerably lower than that of IIV (expressed as variance): 0.0195 vs. 0.0479 for CL and 0.00475 vs. 0.0153 for Vc. Since the inclusion of IOV had no effect on population estimates of Vc or CL, these parameters were not included in the model.
Standard errors of the base model were good for all parameters (CV < 12.1% for fixed effects and < 22.2% for random effects).
3.3 Covariate Analysis and Final Model
Based on the univariate analysis of covariate effects on CL and Vc, VWF level and age, in addition to LBW, were eligible for inclusion in the multivariate analysis. A stepwise deletion process indicated that the best covariate model had an LBW effect on CL and Vc and a VWF effect on CL.
Finally, LBW relationships were replaced through allometric scaling, as recommend by the European Medicines Agency (EMA). As this resulted in a considerable increase of OFV (158 points) and the magnitude of unexplained IIV in Vc more than doubled, LBW was preferred. This finding is in line with the recent study by McEneny-King et al. [15].
Inclusion of a VWF effect on CL slightly decreased the unexplained IIV, from 32.7 to 28.3%. There was no significant relationship between CL or Vc and race or geographic region (Asia vs. other). After inclusion of the LBW and VWF covariates, no visible effects of the non-included continuous covariates remained. Inclusion of a covariance term between CL and Vc resulted in a considerable decrease in OFV (ΔOFV − 29.35) and accounted for the observed correlation between CL and Vc. After exclusion of seven patients with anti-PEG antibodies and/or perceived loss of efficacy and one patient with a drug hypersensitivity reaction, the IIV estimate on CL decreased (from 28.3 to 23.7%) and the covariate effect estimate of LBW on CL increased (from 0.611 to 0.707). However, VPCs showed that excluding data from these eight patients did not affect the model’s predictive ability in any of the age quartiles. Thus, the resulting model in which patients with anti-PEG antibodies, perceived loss of efficacy, or drug hypersensitivity were excluded was considered the final model. Bland–Altman plots compared FVIII activity measured by both assays (Fig. 4). The final model was evaluated by both chromogenic and one-stage assay data, and the pharmacokinetic model parameters were found to be similar.
Bland–Altman plot showing differences in measured BAY 94-9027 factor VIII (FVIII) activity between one-stage and chromogenic assay results based on the phase I and PROTECT VIII studies. Dashed blue horizontal lines at y = 0 correspond to perfect agreement between assay results. Solid (dashed) red horizontal lines indicate the median (upper and lower 95% range) of differences between assays. Blue-shaded areas indicate the 95% confidence interval of the Loess regression. Top panel: all data shown in one plot, with dashed blue vertical lines representing concentration bins; all data are shown excluding outliers (upper and lower 0.5% of samples) for readability. Bottom panel: data separated into four equally sized bins as indicated in the top panel; only samples with FVIII activity above the LLOQ with both assays are shown. Deviation between assays and absolute noise increased with increasing reference value (defined as mean). As the reference increased, one-stage assay results were also more likely to be higher than observations from the chromogenic assay. At lower reference values, there was little bias and no structural trend. At trough levels, 50% of data deviated from the reference value by < 1 IU/dL, with 95% of data within ± 4.1 IU/dL. The final model was evaluated by both chromogenic and one-stage assay data, and the pharmacokinetic model parameters were found to be similar for both assays. Q quartile
In the final model based on chromogenic assay data, CL was non-linearly related to LBW with an estimated exponent of 0.707 (95% CI 0.597–0.817) and to VWF with an estimated exponent of − 0.604 (95% CI − 0.725 to − 0.483); Vc was non-linearly related to LBW with an estimated exponent of 0.887 (95% CI 0.839–0.935). Standard errors were good for all parameters (CV < 10.2% for fixed effects and < 27.6% for random effects; Table 2). The condition number of the model was 15.3, indicating the model was stable and without collinearity in its parameters. Low to moderate shrinkage estimates for the stochastic components (between-subject variability [BSV] CL 15.8, BSV Vc 16.1; RUV 6.13) indicate reliable quantification of the respective variability terms.
The adapted model adequately described the one-stage assay data and precise estimation of parameters (Table 2). VPCs indicated that median FVIII activity in the final model was well-described across all timepoints (Fig. 5), weight, and age groups.
Visual predictive check for the final model and the entire pharmacokinetic dataset. a Comparison of model-predicted median, upper and lower 10th percentile with observations. b Comparison model-predicted median, upper and lower 10th percentile with respective percentiles of the observed data. BLQ below lower limit of quantification, LLOQ lower limit of quantification
3.4 Evaluation of Sparse Sampling Design Strategies
Estimates of CL and Vc using the final popPK model indicated very little general bias introduced by using sparse sampling (Fig. 6). When normalized against estimates based on dense sampling profiles, the median biases of CL and Vc were close to 1 (in fold change), with 95% CIs including 1, for all sampling scenarios. When only one sample was used, the most precise estimate was achieved at 48 h with bias of both pharmacokinetic parameters < 18% for 90% of profiles. A slightly higher precision was observed with use of the Björkman design, which indicated no general bias and < 12% bias in CL and Vc for 90% of samples. Use of a two-sample reduced-Björkman design of 4- and 48-h samples provided robust results identical to the Björkman design.
Box and whisker plot demonstrating the robustness of sparse sampling designs for estimation of BAY 94-9027 pharmacokinetics [14]. Pink and green boxes indicate bias in estimates of clearance (CL) and volume (V), respectively. Notches within the boxes indicate the 95% confidence interval (CI) of the median. Values above the boxes indicate percentages of sparse samples that were within the 95% CIs of the reference parameters based on dense profiles (top value, CL; bottom value, V)
3.5 Simulation Results
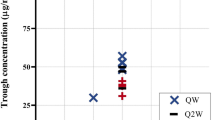
Pharmacokinetic parameter estimates for the 198 patients included in the final model are shown in Table 3. The time between administration of a single BAY 94-9027 dose and the first simulated FVIII value below threshold values of 1, 3, or 5 IU/dL for patients aged ≥ 12 years is shown in Table 4. Predicted median time to reach threshold FVIII level 1 IU/dL following a single BAY 94-9027 dose of 45–60 IU/kg in patients aged 12 to < 18 years and ≥ 18 years ranged from 5.0 to 5.2 and 5.0 to 5.3 days, respectively (Table 4). Simulated patients aged ≥ 12 years whose FVIII levels were above threshold values of 1, 3, or 5 IU/dL at steady state after BAY 94-9027 doses administered once weekly, twice weekly, or every 5 days are shown in Table 5. These simulations predicted that 11.3% and 18.6% of patients aged 12 to < 18 years and ≥ 18 years, respectively, could maintain FVIII levels ≥ 1 IU/dL with once-weekly BAY 94-9027 60 IU/kg.
4 Discussion
In general terms, more favorable profiles would be expected in lean patients with high VWF levels, and dosing based on LBW or similar metrics such as ideal body weight is expected to produce postinfusion levels closer to the theoretical recovery [16].
The model described the data well in patients of different ages and weights, making it suitable for simulation purposes. Although the CL–VWF relationship has been established in the absence of pediatric VWF level data, the levels observed in adult/adolescent patients covered a relatively wide range (47–366 IU/dL), which includes the range typically observed in children [17]; hence, it is reasonable to expect that the identified CL–VWF relationship can be applied across the entire age range. Robust identification of the pharmacokinetics of patients dosed with BAY 94-9027 could be achieved with a single sample collected 48 h postinfusion (low risk of BLQ sample), with particularly low bias observed for Vc because of the informative LBW covariate. Robustness of the estimates was improved by the addition of a second sample collected 4 h postinfusion, leading to identical performance to that observed with the Björkman design. The ideal timepoint for collection of the second sample is likely not limited to 4 h and may provide similar improvements within 2–12 h postinfusion. If only one sample is used, then the potential effects of pre-analytical factors (e.g., sampling or handling) and increased residual variability in a non-study setting should be considered. Nevertheless, dense blood sampling is unlikely to be necessary after administration of BAY 94-9027.
The covariate relationships identified in the BAY 94-9027 popPK model are in line with those of popPK models for other FVIII products [18,19,20]. For BAY 81-8973 (Kovaltry®, Bayer, Berkeley, CA, USA), the popPK model was a two-compartment model, in which LBW was strongly correlated with CL and Vc [5].
BAY 94-9027 pharmacokinetics were adequately described using a one-compartment model, in contrast to antihemophilic factor (recombinant) PEGylated (Adynovate®, Baxalta US Inc., Westlake Village, CA, USA) [21, 22]. PEGylation increases the size of the FVIII molecule, potentially protecting BAY 94-9027 from rapid initial distribution and elimination, and therefore allows higher FVIII levels to be maintained for longer.
When used in conjunction with an assessment of patient characteristics such as age, bleeding profile, and physical activity level [1, 4], determining a patient’s FVIII pharmacokinetics allows an individualized approach to prophylaxis dosing. Weight-based dosing can address some of the variability seen among patients, but pharmacokinetic-based dosing may provide a more finely tuned approach. However, in the absence of pharmacokinetic-based tailored dosing, and keeping within recommended dosing guidelines, dosing based on LBW or on ideal body weight rather than actual body weight can provide effective protection from bleeds [23,24,25] and can be cost-effective for obese patients by anticipating the desired trough level more adequately [23, 26].
Challenges with pharmacokinetic-based dosing occur because of the burden for patients, caregivers, and clinicians of performing the dense blood sampling recommended for traditional pharmacokinetic evaluations and the complexity of the subsequent pharmacokinetic calculations [27, 28]. The ISTH SSC has recommended taking 10–11 blood samples over 32–48 h for adequate pharmacokinetic assessment of standard-half-life FVIII products [29]. With a popPK model, individual pharmacokinetic parameters can be determined based on patient characteristics and using few blood samples [28].
The availability of dedicated, interactive popPK databases, such as the Web-Accessible Population Pharmacokinetic Service-Hemophilia (WAPPS-Hemo), for use with limited blood samples can facilitate popPK evaluations in clinical practice. The WAPPS-Hemo popPK tool was developed using individual pharmacokinetic data provided by manufacturers of coagulation factor products, independent researchers, and hemophilia treatment centers worldwide, providing validated product-specific popPK models [30, 31].
The potential limitation of our popPK model is that it requires external validation and further exploration of its predictive power using few blood samples at varying timepoints.
The longer FVIII levels remain below the therapeutic FVIII level, the greater the risk of breakthrough bleeding episodes during prophylaxis [32]. Using our popPK model, we could estimate time that patients aged ≥ 12 years could maintain FVIII levels above a certain threshold after receiving single BAY 94-9027 25–60 IU/kg doses and predict the percentage of patients who could maintain adequate FVIII levels with BAY 94-9027 prophylaxis once weekly, twice weekly, or every 5 days. These simulated results support the different treatment options for a patient with BAY 94-9027, provide guidance for BAY 94-9027 dosing in clinical practice, and can be used by health economists to estimate BAY 94-9027 product use. Referring to the simulations presented by Shah et al. [33] for adult patients, the median time to reach FVIII threshold levels of 1 IU/dL after a single 50 IU/kg dose was reported to be 3.9 and 3.1 days, respectively, for the standard-half-life products BAY 81-8973 and antihemophilic factor (recombinant) plasma/albumin-free method (rAHF-PFM)—substantially shorter than for BAY 94-9027 (5.1 days; Table 4). Assuming the same schedule and dosing, considerably more patients are expected to maintain FVIII activity above critical threshold levels (Table 5); the proportion of patients predicted to maintain levels always above 1 IU/dL increased by two- to sixfold compared with BAY 81-8973 (27.9%) and rAHF-PFM (6.9%). Therefore, better protection against bleeds would be expected. Future simulation studies could explore aspects of pharmacokinetic-tailored treatment with BAY 94-9027, such as the optimal doses required to consistently maintain patients above important threshold levels.
The residual error was slightly higher than in previous studies for rFVIII products [5, 17]. RUV typically arises from heterogeneity between studies or sites, sampling design, documentation, and handling, the influence of which cannot easily be assessed separately. Further model-based attempts have been conducted to clarify the high unexplained residual variability; these have indicated that the estimate is mainly driven by a small subset of patients not affecting the results and conclusion of the analysis. The sampling design evaluation demonstrated that the relatively high residual error does not interfere with the robust identification of individual pharmacokinetic parameters.
5 Conclusions
BAY 94-9027 is an extended-half-life rFVIII product approved for prophylaxis and on-demand treatment of bleeding episodes in patients with hemophilia A. Determining the optimal dose and dosing frequency for maintaining desired FVIII levels in individual patients remains a challenge for prophylaxis. Pharmacokinetic-based prophylaxis dosing relies on a patient’s FVIII pharmacokinetic profile to individualize the dosing regimen, but traditional pharmacokinetic studies require multiple blood samples. Using data from three BAY 94-9027 clinical trials, we developed a popPK model in which BAY 94-9027 pharmacokinetics were adequately described by a one-compartment model that can be reliably applied for profiling individual BAY 94-9027 pharmacokinetics using sparse postinfusion blood samples. Simulation data using the popPK model confirmed that BAY 94-9027 is an extended-half-life rFVIII with the possibility of tailored prophylaxis using once-weekly, twice-weekly, or every-5-days dosing to target desired trough levels in the range of 1–3 IU/dL.
References
Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Treatment Guidelines Working Group on Behalf of The World Federation of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47.
Iorio A, Marchesini E, Marcucci M, Stobart K, Chan AK. Clotting factor concentrates given to prevent bleeding and bleeding-related complications in people with hemophilia A or B. Cochrane Database Syst Rev. 2011;(9):CD003429.
Barnes C. Importance of pharmacokinetics in the management of hemophilia. Pediatr Blood Cancer. 2013;60(suppl 1):S27–9.
Carcao MD, Iorio A. Individualizing factor replacement therapy in severe hemophilia. Semin Thromb Hemost. 2015;41(8):864–71.
Garmann D, McLeay S, Shah A, Vis P, Maas Enriquez M, Ploeger BA. Population pharmacokinetic characterization of BAY 81-8973, a full-length recombinant factor VIII: lessons learned—importance of including samples with factor VIII levels below the quantitation limit. Haemophilia. 2017;23(4):528–37.
Iorio A, Blanchette V, Blatny J, Collins P, Fischer K, Neufeld E. Estimating and interpreting the pharmacokinetic profiles of individual patients with hemophilia A or B using a population pharmacokinetic approach: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15(12):2461–5.
Iorio A, Edginton AN, Blanchette V, Blatny J, Boban A, Cnossen M, et al. Performing and interpreting individual pharmacokinetic profiles in patients with hemophilia A or B: rationale and general considerations. Res Pract Thromb Haemost. 2018;2(3):535–48.
Coyle TE, Reding MT, Lin JC, Michaels LA, Shah A, Powell J. Phase I study of BAY 94-9027, a PEGylated B-domain-deleted recombinant factor VIII with an extended half-life, in subjects with hemophilia A. J Thromb Haemost. 2014;12(4):488–96.
Reding MT, Ng HJ, Poulsen LH, Eyster ME, Pabinger I, Shin HJ, et al. Safety and efficacy of BAY 94-9027, a prolonged-half-life factor VIII. J Thromb Haemost. 2017;15(3):411–9.
Santagostino E, Saxena K, Kenet G, Fischer K, Biss T, Radke S, et al. PROTECT VIII Kids trial results: BAY 94-9027 safety and efficacy in previously treated children with severe hemophilia A [abstract]. WFH World Congress 2016; 24–28 Jul 2016; Orlando.
US FDA. Jivi® prescribing information. 2018. https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Package-Insert-JIVI.pdf. Accessed 01 Nov 2019.
Wahlby U, Jonsson EN, Karlsson MO. Comparison of stepwise covariate model building strategies in population pharmacokinetic-pharmacodynamic analysis. AAPS PharmSci. 2002;4(4):E27.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Björkman S. Limited blood sampling for pharmacokinetic dose tailoring of FVIII in the prophylactic treatment of haemophilia A. Haemophilia. 2010;16(4):597–605.
McEneny-King A, Chelle P, Foster G, Keepanasseril A, Iorio A, Edginton AN. Development and evaluation of a generic population pharmacokinetic model for standard half-life factor VIII for use in dose individualization. J Pharmacokinet Pharmacodyn. 2019. https://doi.org/10.1007/s10928-019-09634-7(Epub 2019 May 18).
McEneny-King A, Iorio A, Foster G, Edginton AN. The use of pharmacokinetics in dose individualization of factor VIII in the treatment of hemophilia A. Expert Opin Drug Metab Toxicol. 2016;12(11):1313–21.
Appel IM, Grimminck B, Geerts J, Stigter R, Cnossen MH, Beishuizen A. Age dependency of coagulation parameters during childhood and puberty. J Thromb Haemost. 2012;10:2254–63.
Nestorov I, Neelakantan S, Ludden TM, Li S, Jiang H, Rogge M. Population pharmacokinetics of recombinant factor VIII Fc fusion protein. Clin Pharmacol Drug Dev. 2015;4(3):163–74.
Zhang Y, Roberts J, Tortorici M, Veldman A, St Ledger K, Feussner A, et al. Population pharmacokinetics of recombinant coagulation factor VIII-SingleChain in patients with severe hemophilia A. J Thromb Haemost. 2017;5(6):1106–14.
Björkman S, Oh M, Spotts G, Schroth P, Fritsch S, Ewenstein BM, et al. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood. 2012;119(2):612–8.
US FDA. Adynovate. Summary basis for regulatory action. 2016. https://wayback.archiveit.org/7993/20170112211013/http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM535794.pdf. Accessed 01 Nov 2018.
US FDA. Adynovate® prescribing information. 2016. https://www.fda.gov/media/94470/download. Accessed 01 Nov 2019.
McEneny-King A, Chelle P, Henrard S, Hermans C, Iorio A, Edginton AN. Modeling of body weight metrics for effective and cost-efficient conventional factor VIII dosing in hemophilia A prophylaxis. Pharmaceutics. 2017;9(4):E47.
Kidwell AP, Bhatt N, Simpson ML, Boggio LN. Dosing factor in pediatric and adult hemophilia patients using ideal body weight versus actual body weight [abstract]. Blood. 2016;128(22):4958.
Henrard S, Speybroeck N, Hermans C. Impact of being underweight or overweight on factor VIII dosing in hemophilia A patients. Haematologica. 2013;98(9):1481–6.
Graham A, Jaworski K. Pharmacokinetic analysis of anti-hemophilic factor in the obese patient. Haemophilia. 2014;20(2):226–9.
Berntorp E. If you know you will also see: population pharmacokinetics is the way to personalize and optimize prophylaxis in hemophilia. J Thromb Haemost. 2017;15(6):1103–5.
Iorio A. Using pharmacokinetics to individualize hemophilia therapy. Hematology Am Soc Hematol Educ Program. 2017;2017(1):595–604.
Lee M, Morfini S, Schulman S, Ingerslev J. The Factor VIII/Factor IX Scientific and Standardization Committee of the International Society for Thrombosis and Haemostasis. Scientific and Standardization Committee Communication. The design and analysis of pharmacokinetic studies of coagulation factors. 2001. http://c.ymcdn.com/sites/www.isth.org/resource/group/d4a6f49a-f4ec-450f-9e0f-7be9f0c2ab2e/official_communications/fviiipharmaco.pdf. Accessed 19 Feb 2018.
Iorio A, Keepanasseril A, Foster G, Navarro-Ruan T, McEneny-King A, Edginton AN, WAPPS-Hemo Co-Investigator Network, et al. Development of a Web-Accessible Population Pharmacokinetic Service-Hemophilia (WAPPS-Hemo): study protocol. JMIR Res Protoc. 2016;5(4):e239.
McEneny-King A, Foster G, Iorio A, Edginton AN. Data analysis protocol for the development and evaluation of population pharmacokinetic models for incorporation into the Web-Accessible Population Pharmacokinetic Service—Hemophilia (WAPPS-Hemo). JMIR Res Protoc. 2016;5(4):e232.
Collins PW, Blanchette VS, Fischer K, Björkman S, Oh M, Fritsch S, rAHF-PFM Study Group, et al. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7(3):413–20.
Shah A, Solms A, Garmann D, Katterle Y, Avramova V, Simeonov S, et al. Improved pharmacokinetics with BAY 81-8973 versus antihemophilic factor (recombinant) plasma/albumin-free method: a randomized pharmacokinetic study in patients with severe hemophilia A. Clin Pharmacokinet. 2017;56(9):1045–55.
Acknowledgements
Medical writing assistance was provided by Karen L. Zimmermann from Complete Healthcare Communications, LLC (North Wales, PA, USA) and was fully funded by Bayer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Bayer.
Conflict of interest
Alexander Solms, Anita Shah, and Dirk Garmann are employees of Bayer. Alfonso Iorio has received project-based funding via research or service agreements with Bayer, Bioverativ, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, and Shire. Maurice J. Ahsman and Peter Vis were compensated by Bayer for analysis of study data. Erik Berntorp has received research funding from Bayer, Bioverativ, Shire, and Sobi; honoraria for lectures and advisory boards from Bayer, CSL Behring, Grifols, LFB, Octapharma, Pfizer, and Shire; and consultancy fees from Bayer.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Solms, A., Iorio, A., Ahsman, M.J. et al. Favorable Pharmacokinetic Characteristics of Extended-Half-Life Recombinant Factor VIII BAY 94-9027 Enable Robust Individual Profiling Using a Population Pharmacokinetic Approach. Clin Pharmacokinet 59, 605–616 (2020). https://doi.org/10.1007/s40262-019-00832-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00832-7