Abstract
Objective
The pharmacokinetics of nimodipine following enteral administration in the early phase after subarachnoid haemorrhage (SAH) has not been described. If a sufficient absorption could be achieved with enterally administered nimodipine, this would be more feasible dosage form and result in a significant reduction in pharmaceutical costs given that the parenteral formulation of nimodipine currently used is tenfold more expensive than the enteral formulation.
Methods
This was a pilot study in which 17 patients with aneurysmal SAH were randomly assigned to receive nimodipine within 24 h after initial bleeding either as an 60 mg tablet/suspension at 4-h intervals, or as a continuous intravenous infusion of 2 mg/h. Serum nimodipine concentrations were measured during the 4 h following the first dose, and at 24 and 72 h on a validated gas chromatography mass spectrometer (GC-MS).
Results
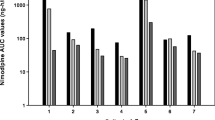
Nimodipine AUC values (expressed in μg min/ml) were lower in the eight SAH patients receiving enteral nimodipine [AUC0–4 range: 0.13–5.4 (median: 0.32); AUC24–28 range: 0.16–6.1 (0.71); AUC72–76 range: 0.47–20.6 (1.9)] than in the nine patients receiving a continuous intravenous infusion of nimodipine [AUC0–4 range: 2.4–4.9 (3.4), p = 0.059; AUC24–28 range: 4.7–10.3 (7.3), p = 0.001; AUC72–76 range: 3.4–8.6 (6.9), p = 0.001]. In three of five good-grade SAH patients receiving nimodipine tablets the AUC values were comparable to those of the intravenous administration, but in two good-grade patients with tablets and in all three poor-grade (Hunt&Hess, grade IV) SAH patients receiving the suspension, the rate and extent of nimodipine absorption was negligible.
Conclusion
This pilot study indicates that the rate and extent of nimopidine absorption following enteral administration in some acute SAH patients could be negligible, and this may particularly be the case in patients with a decreased level of consciousness.



Similar content being viewed by others
References
Brilstra EH, Rinkel GJ, Algra A, van Gijn J (2000) Rebleeding, secondary ischemia, and timing of operation in patients with subarachnoid hemorrhage. Neurology 55:1656–1660
Findlay JM, Deagle GM (1998) Causes of morbidity and mortality following intracranial aneurysm rupture. Can J Neurol Sci 25:209–215
Kassell NF, Torner JC, Haley EC Jr., Jane JA, Adams HP, Kongable GL (1990) The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg 73:18–36
Weir B, MacDonald L (1993) Cerebral vasospasm. Clin Neurosurg 40:40–55
Pickard JD, Murray GD, Illingworth R, Shaw MD, Teasdale GM, Foy PM, Humphrey PRD, Lang DA, Nelson R, Richards P, Sinar J, Bailey S, Skene A (1989) Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ 298:636–642
Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, van Gijn J (2005) Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 1:CD000277
Allen GS, Ahn HS, Preziosi TJ (1983) Cerebral arterial spasm-a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med 308:519–524
Auer LM (1984) Acute operation and preventive nimodipine improve outcome in patients with ruptured cerebral aneurysms. Neurosurgery 15:57–66
Ljunggren B, Brandt L, Säveland H, Nilsson PE, Cronqvist S, Andersson KE, Vinge E (1984) Outcome in 60 consecutive patients treated with early aneurysm operation and intravenous nimodipine. J Neurosurg 61:864–873
Langley MS, Sorkin EM (1989) Nimodipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in cerebrovascular disease. Drugs 37:669–699
Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28:14–20
Helin-Tanninen M, Naaranlahti T, Kontra K, Wallenius K (2001) Enteral suspension of nifedipine for neonates. Part 1. Formulation of nifedipine suspension for hospital use. J Clin Pharm Ther 26:49–57
U.S. Department of Health and Human Services FDA (2001) FDA guidance for industry, bioanalytical method validation. U.S. Department of Health and Human Services FDA, Washington D.C.
Di Pasquale G, Andreoli A, Lusa AM, Urbinati S, Biancoli S, Cere E, Borgatti ML, Pinelli G (1998) Cardiologic complications of subarachnoid hemorrhage. J Neurosurg Sci 42[Suppl 1]:33–36
Vespa PM, Bleck TP (2004) Neurogenic pulmonary edema and other mechanisms of impaired oxygenation after aneurysmal subarachnoid hemorrhage. Neurocrit Care 1:157–170
Koivisto T, Vapalahti M, Parviainen I, Takala J (2001) Gastric tonometry after subarachnoid hemorrhage. Intens Care Med 27:1614–1621
Vinge E, Andersson K-E, Brandt L, Ljunggren B, Nilsson L-G, Rosendal-Helgesen S (1986) Pharmacokinetics of nimodipine in patients with aneurysmal subarachnoid haemorrhage. Eur J Clin Pharmacol 30:421–425
Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, Sternau LL, Torner J, Adams HP Jr, Feinberg W, Thies W (1994) Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 25:2315–2328
Suarez JI, Tarr RW, Selman WR (2006) Aneurysmal subarachnoid hemorrhage. Current concepts. N Eng J Med 354:387–396
Claassen J, Peery S, Kreiter KT, Hirsch LJ, Du EY, Connolly ES, Mayer SA (2003) Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology 60:208–214
Muck W, Ahr G, Kuhlmann J (1995) Nimodipine. Potential for drug-drug interactions in the elderly. Drugs Aging 6:229–242
Acknowledgements
This study was financially supported by an EVO grant from Kuopio University Hospital. The persons contributing to this study do not have any specific conflict of interest. The technical help of laboratory assistant Anne Riekkinen, MSc (Pharmacology), Kirsi Kontra and Toivo Naaranlahti PhD (Pharmacology) is appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soppi, V., Kokki, H., Koivisto, T. et al. Early-phase pharmacokinetics of enteral and parenteral nimodipine in patients with acute subarachnoid haemorrhage – a pilot study. Eur J Clin Pharmacol 63, 355–361 (2007). https://doi.org/10.1007/s00228-007-0267-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-007-0267-7