Abstract
Background
Oral nimodipine is used for prophylaxis and treatment of delayed cerebral ischemia in patients with aneurysmal or perimesencephalic subarachnoid hemorrhage (SAH). In cases of serious refractory cerebral vasospasm, a continuous intra-arterial (IA) infusion of nimodipine (CIAN) may be required to avoid cerebral ischemia. Nimodipine can cause arterial hypotension requiring either a dosage reduction or its discontinuation. Aim of the present study was to examine the effect of different nimodipine formulations on arterial blood pressure in aneurysmal or perimesencephalic SAH patients and to measure the plasma levels after both, peroral administration as tablet or solution and IA infusion.
Methods
In a prospective setting, over a 1-year observation period, data on the course of arterial blood pressure and nimodipine dosage were collected for 38 patients undergoing treatment for aneurysmal or perimesencephalic SAH in an intensive care unit. In addition, plasma concentrations of nimodipine were measured by liquid chromatography–tandem mass spectrometry.
Results
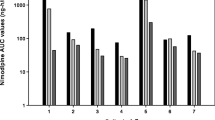
The intended full dose of 60 mg of nimodipine given orally every 4 h could only be administered on 57.2% of the examined days. Ninety-seven episodes of relevant arterial hypotension probably caused by the administration of nimodipine were observed within the first 14 days of treatment. Drops in blood pressure occurred about three times as often after administration of nimodipine as oral solution than as tablet. However, there were no differences in nimodipine plasma levels between the two formulations. In patients suffering from higher-grade SAH, arterial hypotension and consequent dosage reduction or discontinuation of nimodipine were more frequent than in patients with lower-grade SAH. Plasma concentrations of nimodipine during CIAN did not exceed those achieved by oral administration.
Conclusions
Dosage reduction or discontinuation of oral nimodipine is often necessary in patients with higher-grade SAH. Oral nimodipine solutions cause drops in blood pressure more frequently than tablets. Intra-arterial infusion rates of less than 1 mg/h result in venous plasma concentrations of nimodipine similar to those observed after oral application of 60 mg every 4 h.


Similar content being viewed by others
References
Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58.
Dreier JP, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132(Pt 7):1866–81.
Pisapia JM, et al. Microthrombosis after experimental subarachnoid hemorrhage: time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan. Exp Neurol. 2012;233(1):357–63.
Budohoski KP, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012;43(12):3230–7.
Ostergaard L, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013;33(12):1825–37.
Dorhout Mees SM, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007;3:277.
Liu XM, Rinkel GJE. Aneurysmal and clinical characteristics as risk factors for intracerebral haematoma from aneurysmal rupture. J Neurol. 2011;258(5):862–5.
Barker FG, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84(3):405–14.
van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306–18.
Connolly ES, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711–37.
Steiner T, et al. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35(2):93–112.
Diringer MN, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15(2):211–40.
Porchet F, Chiolero R, de Tribolet N. Hypotensive effect of nimodipine during treatment for aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 1995;137(1–2):62–9.
Radhakrishnan D, Menon DK. Haemodynamic effects of intravenous nimodipine following aneurysmal subarachnoid haemorrhage: implications for monitoring. Anaesthesia. 1997;52(5):489–91.
Dankbaar JW, et al. Effect of different components of triple-H therapy on cerebral perfusion in patients with aneurysmal subarachnoid haemorrhage: a systematic review. Crit Care. 2010;14(1):R23.
Boulouis G, et al. Treatment of cerebral vasospasm following aneurysmal subarachnoid haemorrhage: a systematic review and meta-analysis. Eur Radiol. 2017;27(8):3333–42.
Biondi A, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol. 2004;25(6):1067–76.
Cho WS, et al. Intra-arterial nimodipine infusion for cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Interv Neuroradiol. 2011;17(2):169–78.
Kim SS, et al. Angiographic features and clinical outcomes of intra-arterial nimodipine injection in patients with subarachnoid hemorrhage-induced vasospasm. J Korean Neurosurg Soc. 2012;52(3):172–8.
Musahl C, et al. Continuous local intra-arterial nimodipine administration in severe symptomatic vasospasm after subarachnoid hemorrhage. Neurosurgery. 2011;68(6):1541–7.
Wolf S, et al. Continuous selective intraarterial infusion of nimodipine for therapy of refractory cerebral vasospasm. Neurocrit Care. 2010;12(3):346–51.
Bele S, et al. Continuous intra-arterial nimodipine infusion in patients with severe refractory cerebral vasospasm after aneurysmal subarachnoid hemorrhage: a feasibility study and outcome results. Acta Neurochir (Wien). 2015;157(12):2041–50.
Kieninger M, et al. Side effects of long-term continuous intra-arterial nimodipine infusion in patients with severe refractory cerebral vasospasm after subarachnoid hemorrhage. Neurocrit Care. 2018;28(1):65–76.
Vergouwen MD, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–5.
Treggiari MM. Hemodynamic management of subarachnoid hemorrhage. Neurocrit Care. 2011;15(2):329–35.
Darby JM, et al. Acute cerebral blood flow response to dopamine-induced hypertension after subarachnoid hemorrhage. J Neurosurg. 1994;80(5):857–64.
Choi HA, et al. Acute effects of nimodipine on cerebral vasculature and brain metabolism in high grade subarachnoid hemorrhage patients. Neurocrit Care. 2012;16(3):363–7.
Sandow N, et al. Nimodipine dose reductions in the treatment of patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2016;25(1):29–39.
Hanggi D, et al. Newton: nimodipine microparticles to enhance recovery while reducing toxicity after subarachnoid hemorrhage. Neurocrit Care. 2015;23(2):274–84.
Hanggi D, et al. Randomized, open-label, phase 1/2a study to determine the maximum tolerated dose of intraventricular sustained release nimodipine for subarachnoid hemorrhage (Newton [nimodipine microparticles to enhance recovery while reducing toxicity after subarachnoid hemorrhage]). Stroke. 2017;48(1):145–51.
Zussman B, Weiner GM, Ducruet A. Intraventricular nimodipine for aneurysmal subarachnoid hemorrhage: results of the Newton phase 1/2a study. Neurosurgery. 2017;81(1):N3–4.
Hanggi D, et al. Clinical trial protocol: phase 3, multicenter, randomized, double-blind, placebo-controlled, parallel-group, efficacy, and safety study comparing EG-1962 to standard of care oral nimodipine in adults with aneurysmal subarachnoid hemorrhage [Newton-2 (nimodipine microparticles to enhance recovery while reducing toxicity after SubarachNoid hemorrhage)]. Neurocrit Care. 2018. https://doi.org/10.1007/s12028-018-0575-z.
Mohamed S, Riva R, Contin M. Simple and validated UHPLC-MS/MS analysis of nimodipine in plasma and cerebrospinal fluid of patients with subarachnoid haemorrhage. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1028:94–9.
Nirogi RV, et al. Liquid chromatographic-electrospray tandem mass spectrometric method for the quantification of nimodipine in human plasma. Pharmazie. 2006;61(10):828–34.
Vinge E, et al. Pharmacokinetics of nimodipine in patients with aneurysmal subarachnoid haemorrhage. Eur J Clin Pharmacol. 1986;30(4):421–5.
Albanna W, et al. Systemic and cerebral concentration of nimodipine during established and experimental vasospasm treatment. World Neurosurg. 2017;102:459–65.
Abboud T, et al. Serum levels of nimodipine in enteral and parenteral administration in patients with aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien). 2015;157(5):763–7.
Funding
There was no source of funding.
Author information
Authors and Affiliations
Contributions
MK contributed to study concept and design, data collection, data analysis, and manuscript drafting; MG contributed to study concept and design, laboratory work, data analysis, and manuscript drafting; IK carried out study concept and design, data collection, and data analysis; KD performed laboratory work and manuscript revisions; PJO carried out manuscript revisions; SB performed data collection and manuscript revisions; CW collected the data; ST carried out the laboratory work; BG performed manuscript revisions; CE contributed to study concept and design, data collection, data analysis, and critical review of manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Ethical approval and Informed consent
The study was approved and conducted according to the ethical care committee of the University of Regensburg (approval number 16-101-0231). Written informed consent was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kieninger, M., Gruber, M., Knott, I. et al. Incidence of Arterial Hypotension in Patients Receiving Peroral or Continuous Intra-arterial Nimodipine After Aneurysmal or Perimesencephalic Subarachnoid Hemorrhage. Neurocrit Care 31, 32–39 (2019). https://doi.org/10.1007/s12028-019-00676-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00676-w