Abstract
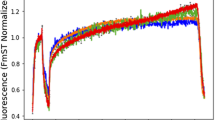
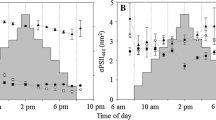
Chlorophyll a fluorescence has been increasingly applied to benthic microalgae, especially diatoms, for measurements of electron transport rate (ETR) and construction of rapid light response curves (RLCs) for the determination of photophysiological parameters [mainly the maximum relative ETR (rETRmax), the light saturation coefficient (E k) and the maximum light use coefficient (α)]. Various problems with the estimation of ETR from the microphytobenthos have been identified, especially in situ. This study further examined the effects of light history of the cells and light dose accumulation during RLCs on the fluorescence measurements of ETR using the benthic diatom Navicula phyllepta. RLCs failed to saturate when using incremental increases in irradiance, however, curves with decreasing irradiance did saturate. Patterns indicating photoacclimation in response to light histories were observed, with higher rETRmax and E k, and lower α, at high light compared to low light. However, these differences could be negated by increasing the RLC irradiance duration from 30 to 60 s. It is suggested that problems arose as a result of rapid fluorescence variations due to ubiquinone (QA) oxidation and non-photochemical chlorophyll fluorescence quenching (NPQ) which depended upon the light history of the cells and the RLCs accumulated light dose. Also, RLCs with irradiance duration of 10 s were shown to have a high level of error possibly specific to the fluorimeter programming. It is suggested that RLCs, using a Diving-PAM fluorimeter on benthic diatoms, should be run using decreasing irradiance steps of 30 s duration.







Similar content being viewed by others
References
Arsalane W, Rousseau B, Duval J-C (1994) Influence of the pool size of the xanthophyll cycle on the effects of light stress in a diatom: competition between photoprotection and photoinhibition. Photochem Photobiol 60:237–243
Barranguet C, Kromkamp J (2000) Estimating primary production rates from photosynthetic electron transport in estuarine microphytobenthos. Mar Ecol Prog Ser 204:39–52
Caron L, Berkaloff C, Duval J-C, Jupin H (1987) Chlorophyll fluorescence transients from the diatom Phaeodactylum tricornutum: relative rates of cyclic phosphorylation and chlororespiration. Photosynth Res 11:131–139
Casper-Lindley C, Björkman O (1998) Fluorescence quenching in four unicellular algae with different light-harvesting and xanthophyll-cycle pigments. Photosynth Res 56:277–289
Consalvey M, Jesus B, Perkins RG, Brotas V, Underwood GJC, Paterson DM (2004) Monitoring migration and measuring biomass in benthic biofilms: the effects of dark/far-red adaptation and vertical migration on fluorescence measurements. Photosynth Res 81:91–101
Consalvey M, Perkins RG, Paterson DM, Underwood GJC (2005a) PAM fluorescence: a beginners guide for benthic diatomists. Diatom Res 20:1–22
Consalvey M, Paterson DM, Underwood GJC (2005b) The ups and downs of life in a benthic biofilm: migration of benthic diatoms. Diatom Res 19:181–202
Dau H (1994) Molecular mechanisms and quantitative models of variable photosystem II fluorescence. Photochem Photobiol 60:1–23
De Brouwer JFC, Wolfstein K, Stal LJ (2002) Physical characterization and diel dynamics of different fractions of extracellular polysaccharides in an axenic culture of a benthic diatom. Eur J Phycol 37:37–44
Dijkman NA, Kroon BMA (2002) Indications for chlororespiration in relation to light regime in the marine diatom Thalassiosira weissflogii. J Photochem Photobiol B 66:179–187
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215
Flameling IA, Kromkamp J (1998) Light dependence of quantum yields for PSII charge separation and oxygen evolution in eucaryotic algae. Limnol Oceanogr 43:284–297
Forster RM, Kromkamp JC (2004) Modelling the effects of chlorophyll fluorescence from subsurface layers on photosynthetic efficiency measurements in microphytobenthic algae. Mar Ecol Prog Ser 284:9–22
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Glud RN, Kühl M, Wenzhöfer F, Rysgaard S (2002) Benthic diatoms of a high Arctic fjord (Young Sound, NE Greenland): importance for ecosystem primary production. Mar Ecol Prog Ser 238:15–29
Harrison PJ, Waters RE, Taylor FJR (1980) A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J Phycol 16:28–35
Hartig P, Wolfstein K, Lippemeier S, Colijn F (1998) Photosynthetic activity of natural microphytobenthos populations measured by fluorescence (PAM) and 14C-tracer methods: a comparison. Mar Ecol Prog Ser 166:53–62
Honeywill C, Paterson DM, Hagerthey SE (2002) Determination of microphytobenthic biomass using pulse amplitude modulated minimum fluorescence. Eur J Phycol 37:485–492
Jakob T, Goss R, Wilhelm C (2001) Unusual pH-dependence of diadinoxanthin de-epoxidase activation causes chlororespiratory induced accumulation of diatoxanthin in the diatom Phaeodactylum tricornutum. J Plant Physiol 158:383–390
Krause GH, Weiss E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Ann Rev Plant Physiol Plant Mol Biol 42:313–349
Kromkamp J, Barranguet C, Peene J (1998) Determination of microphytobenthos PS II quantum efficiency and photosynthetic activity by means of variable chlorophyll fluorescence. Mar Ecol Prog Ser 162:45–55
Kühl M, Glud RN, Borum J, Roberts R, Rysgaard S (2001) Photosynthetic performance of surface-associated algae below sea ice as measured with a pulse-amplitude modulated (PAM) fluorometer and O2 microsensors. Mar Ecol Prog Ser 223:1–14
Lavaud J, Rousseau B, van Gorkom HJ, Etienne A-L (2002a) Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum. Plant Physiol 129:1398–1406
Lavaud J, van Gorkom HJ, Etienne A-L (2002b) Photosystem II electron transfer cycle and chlororespiration in planktonic diatoms. Photosynth Res 74:51–59
Lavaud J, Rousseau B, Etienne A-L (2002c) In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non-photochemical fluorescence quenching. FEBS Lett 523:163–166
Lavaud J, Rousseau B, Etienne A-L (2003) Enrichment of the light-harvesting complex in diadinoxanthin and implications for the non-photochemical fluorescence quenching in diatoms. Biochemistry 42:5802–5808
Lavaud J, Rousseau B, Etienne A-L (2004) General features of photoprotection by energy dissipation in planktonic diatoms (Bacillariophyceae). J Phycol 40:130–137
Lawson T, Oxborough K, Morrison JIL, Baker NR (2002) Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2, and humidity. Plant Physiol 128:52–62
MacIntyre HL, Geider RJ, Miller DC (1996) Microphytobenthos: The ecological role of the ‘secret garden’ of unvegetated, shallow-water marine habitats. I. Distribution, abundance and primary production. Estuaries 19:186–201
MacIntyre HL, Kana TM, Geider RJ (2000) The effect of water motion on short-term rates of photosynthesis by marine phytoplankton. Trends Plant Sci 5:12–17
Mouget J-L, Tremblin G (2002) Suitability of the Fluorescence Monitoring System (FMS, Hansatech) for measurement of photosynthetic characteristics in algae. Aquat Bot 74:219–231
Mouget J-L, Tremblin G, Morant-Manceau A, Morançais M, Robert J-M (1999) Long-term photoacclimation of Haslea ostrearia (Bacillariophyta): effect of irradiance on growth rates, pigment content and photosynthesis. Eur J Phycol 34:109–115
Mouget J-L, Rosa P, Tremblin G (2004) Acclimation of Haslea ostrearia to light of different spectral qualities—confirmation of ‘chromatic adaptation’ in diatoms. J Photochem Photobiol B 75:1–11
Olaizola M, Laroche J, Kolber Z, Falkowski PG (1994) Non-photochemical fluorescence quenching and the diadinoxanthin cycle in a marine diatom. Photosynth Res 41:357–370
Oxborough K, Hanlon ARM, Underwood GJC, Baker NR (2000) An instrument capable of imaging chlorophyll a fluorescence from intact leaves at very low irradiance and at cellular and subcellular levels of organization. Plant Cell Environ 20:1473–1483
Perkins RG, Underwood GJC, Brotas V, Snow G, Jesus B, Ribeiro L (2001) In situ microphytobenthic primary production during low tide emersion in the Tagus estuary, Portugal: production rates, carbon partitioning and vertical migration. Mar Ecol Prog Ser 223:101–112
Perkins RG, Oxborough K, Hanlon ARM, Underwood GJC, Baker NR (2002) Can chlorophyll fluorescence be used to estimate the rate of photosynthetic electron transport within microphytobenthic biofilms? Mar Ecol Prog Ser 228:47–56
Press WH, Teukolsky SA, Vetterling WT, Flannery BP (2003) Numerical recipes in Fortran 77: the art of scientific computing. Cambridge University press, London, p 933
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Bot 82:222–237
Ralph PJ, Gademann R, Larkum AWD, Schreiber U (1999) In situ underwater measurements of photosynthetic activity of coral zooanthellae and other reef-dwelling dinoflagellate endosymbionts. Mar Ecol Prog Ser 180:139–147
Ratkowski DA (1983) Non linear regression modeling. A unified practical approach. Marcal Dekker, New York, p 276
Raven JA, Geider RJ (2003). Adaptation, acclimation and regulation in algal photosynthesis. In: Larkum AWD, Douglas S, Raven JA (eds) Photosynthesis in algae. Adv Photosynth Res 17, Kluwer, Dordrecht, pp 385–412
Ruban AV, Lavaud J, Rousseau B, Guglielmi G, Horton P, Etienne A-L (2004) The super-excess energy dissipation in diatom algae: comparative analysis with higher plants. Photosynth Res 82:165–175
Sakshaug E, Bricaud A, Dandonneau Y, Falkowski P, Keifer D, Legendre L, Morel A, Parslow J, Takahashi M (1997) Parameters of photosynthesis: definitions, theory and interpretation of results. J Plankton Res 19:1637–1670
Schreiber U, Gademann R, Ralph PJ, Larkum AWD (1997) Assessment of photosynthetic performance of Prochloron in Lissoclinum patella in hospite by chlorophyll fluorescence measurements. Plant Cell Physiol 38:945–951
Serôdio J (2003) A chlorophyll fluorescence index to estimate short-term rates of photosynthesis by intertidal microphytobenthos. J Phycol 39:33–46
Serôdio J, Catarino F (2000) Modelling the primary productivity of intertidal microphytobenthos: time scales of variability and effects of migratory rhythms. Mar Ecol Prog Ser 192:13–30
Serôdio J, da Silva JM, Catarino F (1997) Non-destructive tracing of migratory rhythms of intertidal benthic microalgae using in vivo chlorophyll a fluorescence. J Phycol 33:542–553
Serôdio J, Viera S, Cruz S, Barroso F (2005a) Short-term variability in the photosynthetic activity of microphytobenthos as detected by measuring light curves using variable fluorescence. Mar Biol 146:903–914
Serôdio J, Cruz S, Viera S and Brotas V (2005b) Non-photochemical quenching of chlorophyll fluorescence and operation of the xanthophyll cycle in estuarine microphytobenthos. J Exp Mar Biol Ecol 326:144–156
Ting CS, Owens TG (1993) Photochemical and non-photochemical fluorescence quenching processes in the diatom Phaeodactylum tricornutum. Plant Physiol 101:1323–1330
Underwood GJC, Perkins RG, Consalvey MC, Hanlon ARM, Baker NR, Paterson DM (2005) Patterns in microphytobenthic primary productivity: species-specific variation in migratory rhythms and photosynthesis in mixed-species biofilms. Limnol Oceanogr 50:755–767
Wilhelm C, Becker A, Toepel J, Vieler A, Rautenberger R (2004) Photophysiology and primary production of phytoplankton in freshwater. Physiol Plant 120:347–357
Willemoës M, Monas E (1991) Relationship between growth irradiance and the xanthophyll cycle pool in the diatom Nitzschia palea. Physiol Plant 83:449–456
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Thorpe, Port Erin
Rights and permissions
About this article
Cite this article
Perkins, R., Mouget, JL., Lefebvre, S. et al. Light response curve methodology and possible implications in the application of chlorophyll fluorescence to benthic diatoms. Mar Biol 149, 703–712 (2006). https://doi.org/10.1007/s00227-005-0222-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0222-z