Abstract
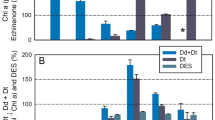
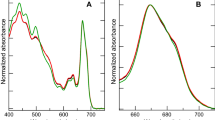
The diadinoxanthin cycle (DD-cycle) in chromophyte algae involves the interconversion of two carotenoids, diadinoxanthin (DD) and diatoxanthin (DT). We investigated the kinetics of light-induced DD-cycling in the marine diatom Phaeodactylum tricornutum and its role in dissipating excess excitation energy in PS II. Within 15 min following an increase in irradiance, DT increased and was accompanied by a stoichiometric decrease in DD. This reaction was completely blocked by dithiothreitol (DTT). A second, time-dependent, increase in DT was detected ∼ 20 min after the light shift without a concomitant decrease in DD. DT accumulation from both processes was correlated with increases in non-photochemical quenching of chlorophyll fluorescence. Stern-Volmer analyses suggests that changes in non-photochemical quenching resulted from changes in thermal dissipation in the PS II antenna and in the reaction center. The increase in non-photochemical quenching was correlated with a small decrease in the effective absorption cross section of PS II. Model calculations suggest however that the changes in cross section are not sufficiently large to significantly reduce multiple excitation of the reaction center within the turnover time of steady-state photosynthetic electron transport at light saturation. In DTT poisoned cells, the change in non-photochemical quenching appears to result from energy dissipation in the reaction center and was associated with decreased photochemical efficiency. D1 protein degradation was slightly higher in samples poisoned with DTT than in control samples. These results suggest that while DD-cycling may dynamically alter the photosynthesis-irradiance response curve, it offers limited protection against photodamage of PS II reaction centers at irradiance levels sufficient to saturate steady-state photosynthesis.
Similar content being viewed by others
Abbreviations
- CAP:
-
chloramphenicol
- D1:
-
PS II reaction center protein
- DD:
-
diadinoxanthin
- DD:
-
cycle-diadinoxanthin cycle
- DT:
-
diatoxanthin
- DTT:
-
dithiothreitol
- FCP:
-
fucoxanthin chlorophyll a-c protein
- Fm :
-
maximum fluorescence yield in the dark-adapted state
- Fo :
-
minimum fluorescence yield in the dark-adapted state
- F′m and F′o :
-
maximum and minimum fluorescence yields respectively in some light adapted state
- Fv :
-
maximum variable fluorescence yield in the dark-adapted state
- Ik :
-
Irradiance at the intercept of the initial slope of the photosynthesis-irradiance curve and the maximum photosynthetic rate
- kD :
-
first order rate constant for nonradiative de-excitation of excitions in the PS II antenna
- kd :
-
first order rate constant for non-radiative de-excitation of excitons in the PS II reaction center
- kF :
-
first order rate constant for fluorescence
- kT :
-
first order rate constant for exciton transfer to the reaction center
- kt :
-
first order rate constant for exciton transfer from the reaction center to the antenna
- Rubisco:
-
ribulose bisphosphate carboxylase
- SVm :
-
Stern-Volmer quenching coefficient of the maximum fluorescence yield
- SVo :
-
Stern-Volmer quenching coefficient of the miniximum fluorescence yield
- σPS II :
-
apparent absorption cross-section of PS II
- τarr :
-
average interval between exciton arrival to the PS II reaction center (ms)
- τrem :
-
average interval between electron turnover during photosynthesis in the PS II reaction center (ms)
- Ψd :
-
the probability that an exciton is non-radiatively dissipated in the reaction center
- ΨT :
-
the probability that an exciton in the antenna is transferred to the reaction center
- Ψt :
-
the probability that an exciton is transferred back from the reaction center to the antenna
References
Adams WW, Demmig-Adams B and Winter K (1990) Relative contributions of zeaxanthin-related and zeaxanthin-unrelated types of ‘high-energy-state’ quenching of chlorophyll fluorescence in spinach leaves exposed to various environmental conditions. Plant Physiol 92: 302–309
Andersson B, Salter AH, Virgin I, Vass I and Styring S (1992) Photodamage to Photosystem II-primary and secondary events. J Photochem Photobiol B: Biol 15: 15–31
Bennett J, Jenkins GI and Hartley MR (1984) Differential regulation of the accumulation of the light-harvesting chlorophyll a/b complex and ribulose bisphosphate carboxylase/oxygenase in greening pea leaves. J Cell Biochem 25: 1–13
Bilger W and Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25: 173–185
Bilger W, Björkman O and Thayer SS (1989) Light-induced spectral absorbance changes in relation to photosynthesis and the epoxidation state of xanthophyll cycle components in cotton leaves. Plant Physiol 91: 542–551
Butler WL (1978) Energy distribution in the photochemical apparatus of photosynthesis. Ann Rev Plant Physiol 29: 345–378
Demers S, Roy S, Gagnon R and Vignault C (1991) Rapid light-induced changes in cell fluorescence and in xanthophyll-cycle pigments of Alexandrium excavatum (Dinophyceae) and Thalassiosira pseudonana (Bacillariophyceae): A photo-protection mechanism. Mar Ecol Progr Ser 76: 185–193
Demmig-Adams B (1990) Carotenoids and photoproteciton in plants: A role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020: 1–24
Demmig-Adams B and Adams WW (1990) The carotenoid zeaxanthin and ‘high-energy-state quenching’ of chlorophyll fluorescence. Photosynth Res 25: 187–197
Demmig-Adams B and Adams WW (1993) The xanthophyll cycle, protein turnover, and the high light tolerance of sun-acclimated leaves. Plant Physiol 103: 1413–1420
Demmig B, Winter K, Krüger A and Czygan F-C (1987) Photoinhibition and zeaxanthin formation in intact leaves. Plant Physiol 84: 218–224
Denman KL and Gargett AE (1983) Time and space scales of vertical mixing and advection of phytoplankton in the upper ocean. Limnol Oceanogr 28: 801–815
Falkowski PG (1983) Light-shade adaptation and vertical mixing of marine phytoplankton: A comparative study. J Mar Res 41: 215–237
Falkowski PG (1992) Molecular ecology of phytoplankton photosynthesis. In: Falkowski PG and Woodhead AD, (eds) Primary Productivity and Biogeochemical Cycles in the Sea, pp 47–67. Plenum Press, New York
Falkowski PG and La Roche J (1991) Molecular biology in studies of ocean processes. Int Rev Cytology 128: 261–303
Falkowski PG, Greene R, Kolber Z (1994) Light utilization and photoinhibition of photosynthesis in marine phytoplankton. In: Baker N and Bowes J (eds) Photoinhibition of Photosynthesis. Bios Scientific, Oxford (in press).
Falkowski PG, Wyman K, Ley AC and Mauzerall DC (1986) Relationship of steady-state photosynthesis to fluorescence in eukaryotic algae. Biochim Biophys Acta 849: 183–192
Falkowski PG Sukenik and Herzig R (1989) Nitrogen limitation in Isochrysis galbana (Haptophyceae). II. Relative abundance of chloroplast protems. J Phycol 25: 471–478
Geider RG, Osborne BA and Raven JA (1986) Growth, photosynthesis and maintenance metabolic cost in the diatom Phaeodactylum tricornutum at very low light levels. J Phycol 22: 39–48
Geider RJ, La Roche J, Greene RM and Olaizola M (1993) Response of the photosynthetic apparatus of Phaeodactylum tricornutum (Bacillariophyceae) to nitrate, phosphate or iron starvation. J Phycol 29: 755–766
Genty B, Harbinson J, Briantais J-M and Baker NR (1990) The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of Photosystem 2 photochemistry in leaves. Photosynth Res 25: 249–257
Giersch C and Krause GH (1991) A simple model relating photoinhibitory fluorescence quenching in chloroplasts to a population of altered Photosystem II reaction centers. Photosynth Res 30: 115–121
Gilmore AM and Yamamoto HY (1991) Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially-mediated linear and cyclic electron transport. Plant Physiol 96: 635–643
Gilmore AM and Yamamoto HY (1992) Dark induction of zeaxanthin-dependent non-photochemical fluorescence quenching mediated by ATP. Proc Natl Acad Sci USA 89: 1899–1903
Gilmore AM and Yamamoto HY (1993) Linear models relating xanthophylls and lumen acidity to non-photochemical fluorescence quenching. Evidence that antheraxanthin explains zeaxanthin-independent quenching. Photosynth Res 35: 67–78
Goldman JC and McCarthy JJ (1978) Steady-state growth and ammonium uptake of a fast growing marine diatom. Limnol Oceanogr 23: 695–703
Greene RM, Geider RJ and Falkowski PG (1991) Effect of iron limitation on photosynthesis in a marine diatom. Limnol Oceanogr 36: 1772–1782
Guillard RRL and Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana (Hustedt) and Detonula confervacea (Cleve). Can J Microbiol 8: 229–239
Hager A (1980) The reversible, light-induced conversions of xanthophylls in the chloroplast. In: Czygan FC (ed) Pigments in Plants, pp 57–79, Fisher, Stuttgart
Kolber Z, Zehr J and Falkowski PG (1988) Effects of growth irradiance and nitrogen limitation on photosynthetic energy conversion in Photosystem II. Plant Physiol 88: 923–929
Krause GH and Weis E (1991) Chlorophyll fluorescence and photosynthesis: The basics. Annu Rev Plant Physiol Plant Mol Biol 42: 313–349
Krieger A, Moya I and Weis E (1992) Energy-dependent quenching of chlorophyll a fluorescence: Effect of pH on stationary fluorescence and picosecond-relaxation kinetics in thylakoid membranes and Photosystem II preparations. Biochim Biophys Acta 1102: 167–176
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227: 680–685
Ley AC and Mauzerall DC (1982) Absolute absorption cross-sections for Photosystem II and the minimum quantum requirements for photosynthesis in Chlorella vulgaris. Biochim Biophys Acta 680: 95–106
Long SP Humphries S and Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Ann Rev Plant Physiol Plant Mol Biol in press
Mantoura RFC and Llewellyn CA (1983) The rapid determination of algal chlorophyll and carotenoid pigments and their breakdown products in natural waters by reverse-phase high-performance liquid chromatography. Anal Chim Acta 151: 297–314
Mauzerall D and Greenbaum NL (1989) The absolute size of a photosynthetic unit. Biochim Biophys Acta 974: 119–140
Mortain-Bertrand A and Falkowski PG (1989) Evidence for a relation between fluorescence and carotenoids: A way to improve models of primary productivity. CR Acad Sci Paris 309: 13–18
Okada K, Satoh K and Katoh S (1991) Chloramphenicol is an inhibitor of photosynthesis. FEBS Lett 295: 155–158
Olaizola M, Bienfang PK and Ziemann DA (1992) Pigment analysis of phytoplankton during a subarctic spring bloom: xanthophyll cycling. J Exp Mar Biol Ecol 158: 59–74
Olaizola M and Yamamoto HY (1994) Short-term response of the diadinoxanthin cycle and fluorescence yield to high irradance in diatoms (Bacillariophyceae). J Phycol 30 (in press)
Ottander C, Hundal T, Andersson, Huner NPA and Öquist G (1993) Photosystem II reaction centres stay intact during low temperature photoinhibition Photosynth Res 35: 191–200
Siefermann-Harms D (1984) Evidence for a heterogeneous organization of violaxanthin in thylakoid membranes. Photochem Photobiol 40: 507–512
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Gocke NM, Olson BJ and Klenk DC (1985) Measurements of protein using Bicinchoninic acid. Anal Biochem 150: 76–85
Sokal RR and Rohlf FJ (1981) Biometry, 2nd ed W.H. Freeman and Co, San Francisco
Sokolave PM and Marscho TV (1976) Ascorbate-independent carotenoid de-epoxidation in intact spinach chloroplasts. Biophys Biochim Acta 430: 321–326
Spurgeon SL and Porter JW (1983) Biosynthesis of carotenoids. In: Porter JW and Spurgeon SL (eds) Biosynthesis of Isoprenoid Compounds, Vol 2, pp 1–122. J Wiley & Sons, New York
Stauber JL and Jeffrey SW (1988) Photosynthetic pigments in fifty-one species of marine diatoms. J Phycol 24: 158–172
Ting CS and Owens TG (1993) Photochemical and non-photochemical fluorescence quenching processes in the diatom Phaeodactylum tricornutum. J Plant Physiol 101: 1323–1330
Towbin H, Staehelin T and Gordon J (1979) Electrophoretic transfer of proteins from polyacrylame gels to nitrocellular sheets. Procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354
Weis E and Berry JA (1987) Quantum effciency of Photosystem II in relation to ‘energy’-dependent quenching of chlorophyll fluorescence. Biochim Biophys Acta 894: 198–208
Yamamoto HY (1985) Xanthophyll cycles. Meth Enzymol 110: 303–312
Yamamoto HY and Kamite L (1972) The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500-nm region. Biochim Biophys Acta 267: 538–543
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Olaizola, M., La Roche, J., Kolber, Z. et al. Non-photochemical fluorescence quenching and the diadinoxanthin cycle in a marine diatom. Photosynth Res 41, 357–370 (1994). https://doi.org/10.1007/BF00019413
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00019413