Abstract
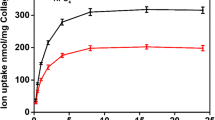
The mineral in bone is located primarily within the collagen fibril, and during mineralization the fibril is formed first and then water within the fibril is replaced with mineral. Our goal is to understand the mechanism of fibril mineralization, and as a first step we recently determined the size exclusion characteristics of the fibril. This study indicates that apatite crystals up to 12 unit cells in size can access the water within the fibril while molecules larger than a 40-kDa protein are excluded. We proposed a novel mechanism for fibril mineralization based on these observations, one that relies exclusively on agents excluded from the fibril. One agent generates crystals outside the fibril, some of which diffuse into the fibril and grow, and the other selectively inhibits crystal growth outside of the fibril. We have tested this mechanism by examining the impact of removing the major serum inhibitor of apatite growth, fetuin, on the serum-induced calcification of collagen. The results of this test show that fetuin determines the location of serum-driven mineralization: in fetuin’s presence, mineral forms only within collagen fibrils; in fetuin’s absence, mineral forms only in solution outside the fibrils. The X-ray diffraction spectrum of serum-induced mineral is comparable to the spectrum of bone crystals. These observations show that serum calcification activity consists of an as yet unidentified agent that generates crystal nuclei, some of which diffuse into the fibril, and fetuin, which favors fibril mineralization by selectively inhibiting the growth of crystals outside the fibril.









Similar content being viewed by others
References
Tong W, Glimcher MJ, Katz JL, Kuhn L, Eppell SJ (2003) Size and shape of mineralites in young bovine bone measured by atomic force microscopy. Calcif Tissue Int 72:592–598
Katz EP, Li S-T (1973) Structure and function of bone collagen fibrils. J Mol Biol 1973:1–15
Sasaki N, Sudoh Y (1996) X-ray pole analysis of apatite crystals and collagen molecules in bone. Calcif Tissue Int 60:361–367
Jager I, Fratzl P (2000) Mineralized Collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys J 79:1737–1748
Landis WJ, Song MJ, Leith A, McEwen L, McEwen BF (1993) Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J Struct Biol 110:39–54
Rubin MA, Jasiuk I, Taylor J, Rubin J, Ganey T, Apkarian RP (2003) TEM analysis of the nanostructure of normal and osteoporotic human trabecular bone. Bone 33:270–282
Robinson RA, Elliott SR (1957) The water content of bone. The mass of water, inorganic crystals, organic matrix, and “CO2 space” components in a unit volume of dog bone. J Bone Joint Surg Am 39:167–188
Boivin G, Meunier P (2002) The degree of mineralization of bone tissue measured by computerized quantitative contact microtomography. Calcif Tissue Int 70:503–511
Toroian D, Lim JE, Price PA (2007) The size exclusion characteristics of type I collagen: implications for the role of non-collagenous bone constituents in mineralization. J Biol Chem 282:22437–22447
Hamlin NJ, Price PA (2004) Mineralization of decalcified bone occurs under cell culture conditions and requires bovine serum but not cells. Calcif Tissue Int 75:231–242
Price PA, June HH, Hamlin NJ, Williamson MK (2004) Evidence for a serum factor that initiates the recalcification of demineralized bone. J Biol Chem 279:19169–19180
Parfitt AM (2000) The mechanism of coupling: a role for the vasculature. Bone 26:319–323
Hamlin NJ, Ong KG, Price PA (2006) A serum factor that recalcifies demineralized bone is conserved in bony fish and sharks but is not found in invertebrates. Calcif Tissue Int 76:326–334
Jahnen-Dechent W, Schinke T, Trindl A, Muller-Esterl W, Sablitzky F, Kaiser S, Blessing M (1997) Cloning and targeted deletion of the mouse fetuin gene. J Biol Chem 272:31496–31503
Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W (1996) The serum protein alpha 2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. J Biol Chem 271:20789–20796
Pedersen KO (1944) Fetuin, a new globulin isolated from serum. Nature 154:575–580
Brown WM, Saunders NR, Mollgard K, Dziegielewska KM (1992) Fetuin—an old friend revisited. Bioessays 14:749–755
Ashton BA, Triffitt JT, Herring GM (1974) Isolation and partial characterization of a glycoprotein from bovine cortical bone. Eur J Biochem 45:525–533
Ashton BA, Hohling HJ, Triffitt JT (1976) Plasma proteins present in human cortical bone: enrichment of the alpha-2HS-glycoprotein. Calcif Tissue Res 22:27–33
Quelch KJ, Cole WG, Melick RA (1984) Noncollagenous proteins in normal and pathological human bone. Calcif Tissue Int 36:545–549
Mizuno M, Farach-Carson MC, Pinero GJ, Fujisawa R, Brunn JC, Seyer JM, Bousfield GR, Mark MP, Butler WT (1991) Identification of the rat bone 60K acidic glycoprotein as alpha 2HS-glycoprotein. Bone Miner 13:1–21
Ohnishi T, Arakaki N, Nakamura O, Hirono S, Daikuhara Y (1991) Purification, characterization, and studies on biosynthesis of a 59-kDa bone sialic acid-containing protein (BSP) from rat mandible using a monoclonal antibody. J Biol Chem 266:14636–14645
Wendel M, Heinegard D, Franzen A (1993) A major non-collagenous 62 kDa protein from rat bone mineralized matrix is identical to pp 63, a phosphorylated glycoprotein from liver. Matrix 13:331–339
Dickson IR, Poole AR, Veis A (1975) Localization of plasma alpha-2-HS glycoprotein in mineralizing human bone. Nature 256:430–432
Price PA, Lim JE (2003) The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin–mineral complex. J Biol Chem 278:22144–22152
Price PA, Chan WS, Jolson DM, Williamson MK (2006) The elastic lamellae of devitalized arteries calcify when incubated in serum. Evidence for a serum calcification factor. Arterioscler Thromb Vasc Biol 26:1079–1085
Price PA, Roublick AM, Williamson MK (2006) Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int doi:10.1038/sj.ki.5001841
Chen PS, Toribara TY, Warner H (1956) Microdetermination of phosphorus. Anal Chem 28:1756–1758
Weiner S, Price PA (1986) Disaggregation of bone into crystals. Calcif Tissue Int 39:365–375
Price PA, Nguyen TMT, Williamson MK (2003) Biochemical characterization of the serum fetuin–mineral complex. J Biol Chem 278:22153–22160
Price PA, June HH, Williamson MK (2003) Evidence for a serum nucleator that initiates the calcification of bone. J Biol Chem
Driessens FCM, Verbeeck RMH (1990) Biominerals. CRC Press, Boca Raton, FL
Elliott JC (1994) Structure and chemistry of the apatites and other calcium orthophosphates. Elsevier, Amsterdam
Bonar L, Roufosse A, WK S, Grynpas M, Glimcher M (1983) X-ray diffraction studies of the crystallinity of bone mineral in newly synthesized and density fractionated bone. Calcif Tissue Int 35:202–209
Meneghini C, Dalconi M, Nuzzo S, Mobilio S, Wenk R (2003) Rietveld refinement on X-ray diffraction patterns of bioapatite in human fetal bones. Biophys J 84:2021–2029
Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W (2003) The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 112:357–366
Westenfeld R, Schafer C, Smeets R, Brandeburg V, Floege J, Ketteler M, Jahnen-Dechent W (2007) Fetuin-A (AHSG) prevents extraosseous calcification induced by uraemia and phosphate challenge in mice. Nephrol Dial Transplant doi:10.1093/ndt/gfm094
Tye CE, Rattray KR, Warner KJ, Gordon JAR, Sodek J, Hunter GK, Goldberg HA (2003) Delineation of the hydroxyapatite-nucleating domains of bone sialoprotein. J Biol Chem 278:7949–7955
Midura R, Wang A, Lovitch D, Law D, Powell K, Gorski J (2004) Bone acidic glycoprotein-75 delineates the extracellular sites of future bone sialoprotein accumulation and apatite nucleation in osteoblastic cultures. J Biol Chem 279:25464–25473
Anderson HC (1995) Molecular biology of matrix vesicles. Clin Orthop Relat Res 314:266–280
Acknowledgement
We thank Gustaf Arrhenius for assistance with the X-ray diffraction analysis. This work was supported in part by grant HL58090 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Toroian, D., Price, P.A. The Essential Role of Fetuin in the Serum-Induced Calcification of Collagen. Calcif Tissue Int 82, 116–126 (2008). https://doi.org/10.1007/s00223-007-9085-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-007-9085-2