Abstract
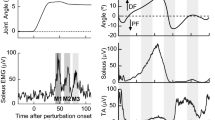
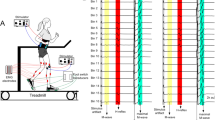
The objective of this study was to assess changes in monosynaptic motoneuron responses to stimulation of Ia afferents after locomotor training in individuals with chronic spinal cord injury (SCI). We hypothesized that locomotor training modifies the amplitude of the soleus monosynaptic motoneuron responses in a body position-dependent manner. Fifteen individuals with chronic clinical motor complete or incomplete SCI received an average of 45 locomotor training sessions. The soleus H-reflex and M-wave recruitment curves were assembled using data collected in both the right and left legs, with subjects seated and standing, before and after training. The soleus H-reflexes and M-waves, measured as peak-to-peak amplitudes, were normalized to the maximal M-wave (M max). Stimulation intensities were normalized to 50 % M max stimulus intensity. A sigmoid function was also fitted to the normalized soleus H-reflexes on the ascending limb of the recruitment curve. After training, soleus H-reflex excitability was increased in both legs in AIS C subjects, and remained unchanged in AIS A-B and AIS D subjects during standing. When subjects were seated, soleus H-reflex excitability was decreased after training in many AIS C and D subjects. Changes in reflex excitability coincided with changes in stimulation intensities at H-threshold, 50 % maximal H-reflex, and at maximal H-reflex, while an interaction between leg side and AIS scale for the H-reflex slope was also found. Adaptations of the intrinsic properties of soleus motoneurons and Ia afferents, the excitability profile of the soleus motoneuron pool, oligosynaptic inputs, and corticospinal inputs may all contribute to these changes. The findings of this study demonstrate that locomotor training impacts the amplitude of the monosynaptic motoneuron responses based on the demands of the motor task in people with chronic SCI.





Similar content being viewed by others
References
Aagaard P, Simonsen EB, Anderson JL, Magnusson P, Dyhre-Poulsen P (2002) Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol 92:2309–2318
Beaumont E, Gardiner P (2002) Effects of daily spontaneous running on the electrophysiological properties of hindlimb motoneurons in rats. J Physiol Lond 540:129–138
Beaumont E, Gardiner PF (2003) Endurance training alters the biophysical properties of hindlimb motoneurons in rats. Muscle Nerve 27:228–236
Beaumont E, Houle JD, Peterson CA, Gardiner PF (2004) Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve 29:234–242
Bennett D, Li Y, Harvey PJ, Gorassini M (2001) Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86:1972–1982
Brinkworth RS, Tuncer M, Tucker KJ, Jaberzadeh S, Türker KS (2007) Standardization of H-reflex analyses. J Neurosci Methods 162:1–7
Burke D, Gandevia SC, McKeon B (1984) Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol 52:435–448
Button DC, Kalmar JM, Gardiner K, Marqueste T, Zhong H, Roy RR, Edgerton VR, Gardiner PF (2008) Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J Physiol Lond 586:529–544
Capaday C (1997) Neurophysiological methods for studies of the motor system in freely moving human subjects. J Neurosci Methods 74:201–218
Capaday C, Stein RB (1987) Difference in the amplitude of the human soleus H-reflex during walking and running. J Physiol Lond 392:513–522
Carroll TJ, Riek S, Carson RG (2001) Reliability of the input–output properties of the cortico-spinal pathway obtained from transcranial magnetic and electrical stimulation. J Neurosci Methods 112:193–202
Cope TC, Bodine SC, Fournier M, Edgerton VR (1986) Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J Neurophysiol 55:1202–1220
Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN (2008) Spinal and supraspinal postural networks. Brain Res Rev 57:212–221
Devanne H, Lavoie BA, Capaday C (1997) Input–output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Dietz V, Colombo G, Jensen L (1994) Locomotor activity in spinal man. Lancet 344:1260–1263
Dietz V, Wirtz M, Curt A, Colombo G (1998) Locomotor pattern in paraplegic patients: training effects and recovery of spinal cord function. Spinal Cord 36:380–390
Dimitrijevic MR, Nathan PW, Sherwood AM (1980) Clonus: the role of central mechanisms. J Neurol Neurosurg Psychiatry 43:321–332
Dobkin BH (2000) Spinal and supraspinal plasticity after incomplete spinal cord injury: correlations between functional magnetic resonance imaging and engaged locomotor networks. Prog Brain Res 128:99–111
Dunlop SA (2008) Activity-dependent plasticity: implications for recovery after spinal cord injury. Trends Neurosci 31:410–418
Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR (2004) Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci 27:145–167
Faist M, Hoefer C, Hodapp M, Dietz V, Berger W, Duysens J (2006) In humans Ib facilitation depends on locomotion while suppression of Ib inhibition requires loading. Brain Res 1076:87–92
Field-Fote EC, Roach KE (2011) Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther 91:48–60
Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG (2004) Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol 476:130–145
Hajela N, Mummidisetty CK, Smith AC, Knikou M (2013) Corticospinal reorganization after locomotor training in a person with motor incomplete paraplegia. Biomed Res Int 2013:516427
Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J (2009) Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol 120:2040–2054
Heckmann CJ, Gorassini MA, Bennett DJ (2005) Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31:135–156
Hochman S, McCrea DA (1994a) Effects of chronic spinalization on ankle extensor motoneurons. I. Composite monosynaptic Ia EPSPs in four motoneuron pools. J Neurophysiol 71:1452–1467
Hochman S, McCrea DA (1994b) Effects of chronic spinalization on ankle extensor motoneurons II. Motoneuron electrical properties. J Neurophysiol 71:1468–1479
Holtermann A, Roeleveld K, Engstrom M, Sand T (2007) Enhanced H-reflex with resistance training is related to increased rate of force development. Eur J Appl Physiol 101:301–312
Hornby TG, Rymer WZ, Benz EN, Schmit BD (2003) Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol 89:416–426
Hornby TG, Zemon DH, Campbell D (2005) Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther 85:52–66
Ilha J, Centenaro LA, Broetto Cunha N, de Souza DF, Jaeger M, do Nascimento PS, Kolling J, Ben J, Marcuzzo S, Wyse AT, Gottfried C, Achaval M (2011) The beneficial effects of treadmill step training on activity-dependent synaptic and cellular plasticity markers after complete spinal cord injury. Neurochem Res 36:1046–1055
Katz R, Meunier S, Pierrot-Deseilligny E (1988) Changes in presynaptic inhibition of Ia fibres in man while standing. Brain 111:417–437
Kernell D, Hultborn H (1990) Synaptic effects on recruitment gain: a mechanism of importance for the input–output relations of motoneurone pools? Brain Res 507:176–179
Klimstra M, Zehr EP (2008) A sigmoid function is the best fit for the ascending limb of the Hoffmann reflex recruitment curve. Exp Brain Res 186:93–105
Knikou M (2007) Hip-phase-dependent flexion reflex modulation and expression of spasms in patients with spinal cord injury. Exp Neurol 204:171–181
Knikou M (2008) The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 171:1–12
Knikou M (2012) Function of group Ib inhibition during assisted stepping in human spinal cord injury. J Clin Neurophysiol 29:271–277
Knikou M (2013) Functional reorganization of soleus H-reflex modulation during stepping after robotic-assisted step training in people with complete and incomplete spinal cord injury. Exp Brain Res 228:279–296
Knikou M, Mummidisetty CK (2011) Reduced reciprocal inhibition during assisted stepping in human spinal cord injury. Exp Neurol 231:104–112
Knikou M, Mummidisetty CK (2014) Locomotor training improves premotoneuronal control after spinal cord injury. J Neurophysiol 111:2264–2275
Knikou M, Angeli CA, Ferreira CK, Harkema SJ (2009) Soleus H-reflex gain, threshold, and amplitude as function of body posture and load in spinal cord intact and injured subjects. Int J Neurosci 119:2056–2073
Lagerquist O, Zehr EP, Docherty D (2006) Increased spinal reflex excitability is not associated with neural plasticity underlying the cross-education effect. J Appl Physiol 100:83–90
Lee JK, Emch GS, Johnson CS, Wrathall JR (2005) Effect of spinal cord injury severity on alterations of the H-reflex. Exp Neurol 196:430–440
Lee HJ, Jakovcevski I, Radonjic N, Hoelters L, Schachner M, Irintchev A (2009) Better functional outcome of compression spinal cord injury in mice is associated with enhanced H-reflex responses. Exp Neurol 216:365–374
Manella KJ, Field-Fote EC (2013) Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci 31:633–646
Mummidisetty CK, Bohórquez J, Thomas CK (2012) Automatic analysis of EMG during clonus. J Neurosci Methods 204:35–43
Mummidisetty CK, Smith AC, Knikou M (2013) Modulation of reciprocal and presynaptic inhibition during robotic-assisted stepping in humans. Clin Neurophysiol 124:557–564
Nicolas G, Marchand-Pauvert V, Burke D, Pierrot-Deseilligny E (2001) Corticospinal excitation of presumed cervical propriospinal neurones and its reversal to inhibition in humans. J Physiol Lond 533:903–919
Nielsen J, Kagamihara Y (1992) The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. J Physiol Lond 456:373–391
Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy RR, Edgerton VR, Mendell LM (2007) Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J Neurosci 27:4460–4471
Pierrot-Deseilligny E, Burke D (2012) Spinal and corticospinal mechanisms of movement. Cambridge University Press, New York
Pierrot-Deseilligny E, Mazevet D (2000) The monosynaptic reflex: a tool to investigate motor control in humans. Interests and limits. Neurophysiol Clin 30:67–80
Rossignol S, Frigon A (2011) Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci 34:413–440
Rossignol S, Barriere G, Alluin O, Frigon A (2009) Re-expression of locomotor function after partial spinal cord injury. Physiol 24:127–139
Smith AC, Mummidisetty CK, Rymer WZ, Knikou M (2014) Locomotor training alters the behavior of flexor reflexes during walking in human spinal cord injury. J Neurophysiol. doi:10.1152/jn.00308.2014
Tan U (1990) There is a close relationship between hand skill and the excitability of motor neurons innervating the postural soleus muscle in left-handed subjects. Int J Neurosci 51:25–34
Thomas SL, Gorassini MA (2005) Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol 94:2844–2855
Trimble MH, Behrman AL, Flynn SM, Thigpen MT, Thompson FJ (2001) Acute effects of locomotor training on overground walking speed and H-reflex modulation in individuals with incomplete spinal cord injury. J Spinal Cord Med 24:74–80
Wallace DM, Ross BH, Thomas CK (2005) Motor unit behavior during clonus. J Appl Physiol 99:2166–2172
Wernig A, Müller S, Nanassy A, Cagol E (1995) Laufband therapy based on rules of spinal locomotion is effective in spinal cord injured persons. Eur J Neurosci 7:823–829
Winchester P, McColl R, Querry R, Foreman N, Mosby J, Tansey K, Williamson J (2005) Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabil Neural Repair 19:313–324
Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, Hornby TG (2005) Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehab 86:672–680
Wolpaw JR (2007) Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol 189:155–169
Yang JF, Fung J, Edamura M, Blunt R, Stein RB, Barbeau H (1991) H-reflex modulation during walking in spastic paretic subjects. Can J Neurol Sci 18:443–452
Zehr EP, Loadman PM, Hundza SR (2012) Neural control of rhythmic arm cycling after stroke. J Neurophysiol 108:891–905
Acknowledgments
Authors thank all volunteers for participating in many experimental and locomotor training sessions, and Chaithanya K. Mummidisetty for his help during data acquisition. This work was supported by grants from The Craig Neilsen Foundation (Grant No. 83607), and from the New York State Department of Health, Wadsworth Center, Spinal Cord Injury Research Board (Grant No. C023690) awarded to Maria Knikou. Andrew C. Smith is supported by NIH T32 HD057845 and by the Foundation for Physical Therapy Promotion of Doctoral Studies. The funding sources had no involvement in study design, data collection, data analysis, data interpretation, and decision to publish.
Conflict of interest
The authors have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
All work was completed at the Rehabilitation Institute of Chicago (Chicago, IL 60611, USA).
Rights and permissions
About this article
Cite this article
Smith, A.C., Rymer, W.Z. & Knikou, M. Locomotor training modifies soleus monosynaptic motoneuron responses in human spinal cord injury. Exp Brain Res 233, 89–103 (2015). https://doi.org/10.1007/s00221-014-4094-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-4094-7