Abstract
In people with spasticity due to chronic incomplete spinal cord injury (SCI), it has been presumed that the abnormal stretch reflex activity impairs gait. However, locomotor stretch reflexes across all phases of walking have not been investigated in people with SCI. Thus, to understand modulation of stretch reflex excitability during spastic gait, we investigated soleus stretch reflexes across the entire gait cycle in nine neurologically normal participants and nine participants with spasticity due to chronic incomplete SCI (2.5–11 year post-injury). While the participant walked on the treadmill at his/her preferred speed, unexpected ankle dorsiflexion perturbations (6° at 250°/s) were imposed every 4–6 steps. The soleus H-reflex was also examined. In participants without SCI, spinal short-latency “M1”, spinal medium latency “M2”, and long-latency “M3” were clearly modulated throughout the step cycle; the responses were largest in the mid-stance and almost completely suppressed during the stance-swing transition and swing phases. In participants with SCI, M1 and M2 were abnormally large in the mid–late-swing phase, while M3 modulation was similar to that in participants without SCI. The H-reflex was also large in the mid–late-swing phase. Elicitation of H-reflex and stretch reflexes in the late swing often triggered clonus and affected the soleus activity in the following stance. In individuals without SCI, moderate positive correlation was found between H-reflex and stretch reflex sizes across the step cycle, whereas in participants with SCI, such correlation was weak to non-existing, suggesting that H-reflex investigation would not substitute for stretch reflex investigation in individuals after SCI.
Similar content being viewed by others
Introduction
Spinal reflexes are often altered after spinal cord injury (SCI) that disrupts the activity of supraspinal and propriospinal pathways (Yang et al. 1991; Stein et al. 1993; Hiersemenzel et al. 2000; Crone et al. 2003; Thompson et al. 2009b). Since the activity of spinal reflex pathways is task- and phase-dependently modulated in functionally relevant ways in normal motor control (Capaday and Stein 1986, 1987a; Stein and Capaday 1988; Duysens et al. 1990; Yang and Stein 1990; Zehr et al. 1997, 1998b; Zehr and Stein 1999b; Duysens et al. 2004) and different spinal pathways contribute to the generation of normal muscle activity during locomotion (Sinkjaer et al. 1996a; Grey et al. 2002, 2007; Mazzaro et al. 2005, 2006), the natural presumption has been that spinal reflex abnormalities contribute to impaired movement control (Dietz and Sinkjaer 2007; Nielsen et al. 2007). In fact, reflex abnormalities in the soleus of people after SCI have been described in multiple studies: absent or diminished task-dependent modulation of the H-reflex (Boorman et al. 1996; Thompson et al. 2009b); absence of Ib inhibition (Morita et al. 2006); abnormal reciprocal inhibition between the soleus and tibialis anterior (Ashby and Wiens 1989; Boorman et al. 1991, 1996; Crone et al. 2003; Thompson et al. 2009b); and increased recurrent inhibition (Shefner et al. 1992). Reflex abnormalities also exist in other muscles (Faist et al. 1999). Furthermore, observations of abnormal reflex behaviors (including motoneuronal and interneuronal activity) during static motor tasks or isolated joint motion (Hultborn 2003; Gorassini et al. 2004; Li et al. 2004; Hornby et al. 2006) have suggested several compelling potential mechanisms of movement disorders. Yet, findings from those studies do not provide direct evidence of reflex malfunctioning during dynamic motion. To better understand a reflex’s function during movement, the reflex must be examined during movement (Stein and Thompson 2006).
Spasticity, commonly characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks (Lance 1980), affects 65–78% of people after SCI (Maynard et al. 1990; Skold et al. 1999; Adams and Hicks 2005) and impairs their locomotion (this could be an over- or under-estimate of the spasticity occurrence, since different symptoms are likely reported as spasticity in clinical practice, including the hyperexcitability of tonic (e.g., hypertonia) and phasic (e.g., clonus) stretch reflexes of intrinsic and extrinsic origins (Decq 2003; Adams and Hicks 2005)). While several spinal and supraspinal pathways are thought to be involved in spastic movement disorders (Hultborn 2003; Dietz and Sinkjaer 2007; Nielsen et al. 2007; Burke et al. 2013; Li and Francisco 2015), how they contribute to gait impairments in SCI is not well understood. In people with SCI, the soleus stretch reflex excitability is often elevated in static postures or during hip rotation (Mirbagheri et al. 2001; Kawashima et al. 2006; Nakazawa et al. 2006), and clonus, hyperactivity, and abnormal modulation of the soleus H-reflex are frequently present (Corcos et al. 1986; Fung and Barbeau 1989, 1994; Yang et al. 1991; Stein et al. 1993; Hidler and Rymer 1999; Barbeau et al. 2002; Knikou et al. 2009a, b; Knikou 2013). Thus, to understand how the excitability of stretch reflex pathways is modulated during spastic gait, here, we investigated the soleus stretch reflexes across the entire gait cycle.
While the H-reflex is commonly viewed as the response generated through the same pathway as the short-latency stretch reflex and often used to investigate Ia excitation of homonymous motoneurons (Zehr 2002; Misiaszek 2003), the short-latency reflex (called M1) and H-reflex do not necessarily respond to modulatory input in the same way (Nielsen et al. 1994; Sinkjaer et al. 1996a; Morita et al. 1998). The H-reflex is an electrically elicited spinal reflex that arises mainly from synchronous axonal excitation of large Ia (and some Ib/II) afferents, whereas the stretch reflexes elicited by rapid joint rotation arise from less synchronous activation of Ia and II afferents and reflect γ-motoneuron mediated fusimotor control (Magladery et al. 1951; Henneman and Mendell 1980; McKeon and Burke 1983; Morita et al. 1998). Ib afferents arising from Golgi tendon organ also fire in response to muscle stretch, and excite motoneurons via interneurons (Lundberg et al. 1978; Jankowska and McCrea 1983; Schafer et al. 1999; Duysens et al. 2000; Jankowska and Edgley 2010; Vincent et al. 2017; Nichols 2018). In sum, while the afferent populations that elicit H-reflexes and stretch reflexes overlap, their compositions are not identical; furthermore, stretch reflex afferent excitation is less synchronous and is affected by fusimotor control of the muscle spindle. Thus, the study of both reflexes in parallel would provide further insight into their activation, modulation, and function during walking. To evaluate correlation and predictability of reflex excitability between the H-reflex and the stretch reflexes during dynamic motion, in this study, we examined across all phases of the gait cycle, the three components of soleus stretch reflexes: spinal short-latency “M1” (mainly Ia afferent origin), spinal medium latency “M2” (presumably mainly II afferent mediated), and long-latency “M3” (suggested to be transcortical or subcortical) responses (Corna et al. 1995; Sinkjaer et al. 1996b; Schieppati and Nardone 1997; Nardone and Schieppati 1998; Andersen and Sinkjaer 1999; Sinkjaer et al. 1999; Grey et al. 2001; Uysal et al. 2009; Af Klint et al. 2010; Uysal et al. 2011), together with the soleus H-reflex.
Materials and methods
Participants
Soleus H-reflex and stretch reflexes were examined in nine participants with no known neurological conditions (seven men and two women; age range 30–68 years; mean ± SD age 46.3 ± 11.9 years) and nine ambulatory participants with spasticity due to chronic incomplete SCI (eight men and one woman; age range 31–68 years; mean ± SD age 52.1 ± 11.3 years; 2.5–11 year post-injury, Table 1). The impairments of these participants fell into the American Spinal Injury Association Impairment Scale (AIS) D (i.e., motor incomplete). All participants gave informed consent for the study, which was reviewed and approved by the Institutional Review Board of Helen Hayes Hospital and the Medical University of South Carolina.
Inclusion criteria for participants with SCI were: (1) clinically stable (> 6 months after lesion); (2) medical clearance to participate; (3) ability to walk on the treadmill at 0.2 m/s or faster for at least 5 min at a time; (4) clinical sign of spasticity (i.e., Modified Ashworth Scale > 1) and/or occurrence of clonus more than once a day at the ankle at least unilaterally. Six of nine participants had been taking a stable dose of baclofen, with or without other anti-spastic medication (e.g., tizanidine and diazepam), for at least 6 months prior to the study. All of them had been medically stable with no change of medication for at least 3 months prior to the day of the experiment. Exclusion criteria were: (1) lower motor neuron injury; (2) known cardiac conditions; (3) medically unstable conditions; (4) cognitive impairment; and (5) extensive use of functional electrical stimulation (FES) foot-drop stimulator on a daily basis. In the participants who exhibited sign of spasticity bilaterally, the leg with more spasticity was investigated.
Study overview
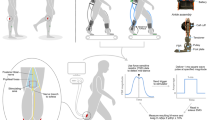
At the beginning of the experiment, electromyography (EMG) recording and tibial nerve stimulating electrodes were placed over the leg, and the soleus H-reflex/M-wave recruitment curve was obtained, while the participant stood and maintained a stable level of soleus background EMG activity (see EMG recording and nerve stimulation). Then, stretch reflexes were elicited by ankle dorsiflexion (6° at 250°/s) during standing with the same stable level of background EMG activity. Following that, locomotor stretch reflexes were measured, while the participant walked on the treadmill at his/her comfortable speed while wearing a portable joint perturbation device (Andersen and Sinkjaer 1995). Ten-to-fifteen dorsiflexions (aimed at 6°–8° at ≈ 250°/s) were applied in each of 8 equal time bins of the step cycle (Fig. 1). Before or after locomotor stretch reflex measurements, the locomotor H-reflex was also measured; soleus H-reflexes were elicited by tibial nerve stimulation at just above M-wave threshold in each of 8 equal time bins of the step cycle (as for the stretch reflex measurement). A typical experiment took ~ 1.5 h.
Examples of stretch reflexes, locomotor EMG activity, and ankle joint motion in a participant without known neurological injuries. aM1, M2, and M3 soleus stretch reflexes elicited by a rapid 6° ankle dorsiflexion (250°/s) during standing. b Ankle joint motion and soleus and TA EMG activity during walking in a normal subject. With eight equal bins of the step cycle starting from foot contact, bins 1–4 correspond to the stance phase, bin 5 to the stance-swing transition, and bins 6–8 to the swing phase
Device to induce stretch reflexes
To induce and measure stretch reflexes, we used a unique joint motion perturbation device that is capable of imposing a fast ankle joint rotation during gait (Andersen and Sinkjaer 1995). This is a two-link system consisting of a mechanical joint which is strapped to the lower leg and foot of the participant. The mechanical joint aligned to the ankle joint rotates through Bowden wires and is connected to a motor placed next to the treadmill on which the participant walks. Using position feedback from the mechanical joint, the motor can dorsiflex the ankle at a specific ankle joint angle. The system is designed to impose a fast joint rotation of up to 20°. This allows, for example, an 8° joint rotation over 30 ms [known to be effective in inducing stretch reflexes (Sinkjaer et al. 1996a, 1999)] to be performed at any point in the gait cycle. The mechanical joint attached to the participant’s leg and foot weighs < 0.9 kg, and is not too heavy for ambulatory individuals who are capable of treadmill walking (Sinkjaer et al. 1996a; Nielsen et al. 1998; Andersen and Sinkjaer 1999; Turner et al. 1999; Mrachacz-Kersting et al. 2004; Willerslev-Olsen et al. 2014). Indeed, despite wearing the mechanical joint, the participants of this study often did not notice the small rapid ankle dorsiflexions applied during walking. This unique joint motion perturbation device (Andersen and Sinkjaer 1995) has been used safely and effectively in many previous studies, and has revealed the activity of stretch reflex pathways during walking in neurologically normal individuals (Sinkjaer et al. 1996a; Andersen and Sinkjaer 1999; Christensen et al. 2000; Sinkjaer et al. 2000; Grey et al. 2001, 2002; Mazzaro et al. 2005) and individuals with multiple sclerosis (Sinkjaer et al. 1996b, 1999), stroke (Nielsen et al. 1998), and cerebral palsy (Willerslev-Olsen et al. 2014).
EMG recording and nerve stimulation
The soleus and its antagonist tibialis anterior (TA) EMG signals were recorded with surface self-adhesive Ag–AgCl electrodes (2.2 × 3.5 cm, Vermed, Inc., Bellows Falls, VT). Pairs of EMG recording electrodes were placed just below the gastrocnemius in line with the Achilles tendon for the soleus, and over the muscle belly for the TA, with their centers 3 cm apart. EMG activity was amplified, bandpass filtered (10–1000 Hz), sampled at 4000 Hz, and stored. One participant who wore an ankle foot orthosis removed it for the reflex measurements.
To elicit the soleus H-reflex, the tibial nerve was stimulated in the popliteal fossa, using surface Ag–AgCl electrodes (2.2 × 2.2 cm for the cathode electrode and 2.2 × 3.5 cm for the anode electrode, Vermed, Inc., Bellows Falls, VT). The stimulating electrode pair was placed so as to minimize the H-reflex threshold, maximize the maximum M-wave size, and avoid stimulation of other nerves.
To elicit the H-reflex and M-wave during standing, 1-ms square stimulus pulses were delivered through a Grass S48 stimulator with an SIU-5 stimulation isolation unit and a CCU1 constant current unit (Natus Neurology—Grass, Warwick, RI) when the participant maintained soleus EMG activity within the specified range [i.e., natural standing level of EMG activity, which typically corresponds to 10–20% of maximum voluntary contraction level of EMG activity (Thompson et al. 2009a)] and the resting level of TA activity (i.e., < 7 μV) for at least 2 s. The minimum interstimulus interval was 5 s. To obtain the soleus H-reflex/M-wave recruitment curve, the tibial nerve stimulus intensity was varied in increments of 1.2–2.5 mA from the H-reflex threshold to the maximum H-reflex (Hmax) to an intensity just above what was needed to elicit the maximum M-wave (Mmax) (Zehr and Stein 1999a; Kido et al. 2004a). Four EMG responses were averaged to measure the H-reflex and M-wave at each intensity, and ~ 10 different intensities were used to obtain the recruitment curve.
To elicit stretch reflexes during standing, 6° of rapid dorsiflexion was applied at 250°/s in the same standing posture as the H-reflex testing, with the same soleus and TA background EMG level requirements and the same minimum interstimulus interval.
For reflex measurements during walking, foot-switch cells were inserted between the participant’s shoe and foot to detect the foot contact of the stimulated leg. Prior to testing reflexes during walking, the participant walked on a treadmill without stimulation for 1–2 min at his/her comfortable speed (wearing a safety harness) to become used to walking with the mechanical joint attached. As noted above, the device did not cause discomfort or restrict the natural joint motion during walking (except when a perturbation was applied). Thus, the device did not correct or otherwise affect any gait abnormalities such as foot drop. Once the participant was comfortable with the procedure, locomotor reflex testing began. Walking speed, which was self-selected differed (p = 0.01, unpaired t test) between participants without injuries [0.91 ± 0.05(mean ± SE) m/s] and participants with SCI (0.62 ± 0.08 m/s).
For H-reflex testing, single 1-ms square pulse stimuli were delivered at different points in the step cycle (Capaday and Stein 1986; Stein and Capaday 1988; Schneider et al. 2000; Ethier et al. 2003; Kido et al. 2004a). The stimulus interval was long enough to ensure at least one full unstimulated step between stimuli. For analysis, the foot-contact signal was used to define the beginning of the step cycle; thus, the step cycle went from the beginning of the stance phase to the end of the swing phase. EMG signals from individual unstimulated steps (typically > 100 steps) were normalized to the mean step cycle time and averaged to obtain the locomotor EMG activity over the step cycle (Kido et al. 2004b; Makihara et al. 2012; Thompson et al. 2013b; Makihara et al. 2014). The step cycle was divided into 8 equal bins and EMG activity was averaged for each bin. To measure the background EMG activity for H-reflex measurement during walking, the average soleus EMG activity was computed for unstimulated steps and used as the control background EMG (Yang and Stein 1990; Kido et al. 2004b; Makihara et al. 2012). The mean rectified H-reflex was calculated as [stimulated EMG − control EMG (of the corresponding time in the step cycle)] in the H-reflex window (typically 30–45 ms after the stimulus). Then, the sizes of the peak-to-peak or mean rectified H-reflexes were plotted vs. the time of the step cycle at which the H-reflex was elicited, sorted into 8 equal time bins of the step cycle, and averaged for each bin (Zehr et al. 1997, 1998a; Kido et al. 2004b; Makihara et al. 2012).
For stretch reflex testing, a rapid 6°–8° of ankle dorsiflexion was produced by the attached device every 4–6 steps. Dorsiflexion perturbations occurred in 8 equal bins of the step cycle: they centered at about 5, 17.5, 30, 42.5, 55, 67.5, 80, and 92.5% of the step cycle (Fig. 1). Note that after imposing dorsiflexion perturbation, the mechanical joint was temporarily locked in the post-perturbation position for ≈ 150 ms, to prevent potential contamination of stretch reflex EMG signals by unlocking of the mechanical joint too soon. This temporally affected gait mechanics in the perturbed step but their normalcy was restored within the following step or two (i.e., before the subsequent perturbation was applied). Ten-to-fifteen dorsiflexion perturbations were applied in each bin. The perturbation device system setting that produced ≈ 250°/s rotation during standing resulted in different rotation speeds during walking; actual ankle rotation speeds (summarized in Table 2) were 273 ± 11 (SD)°/s in participants without injuries and 235° ± 10°/s in participants with SCI, different between the groups (p < 0.0001, unpaired t test). Note that this difference may partly be due to the difference in intrinsic joint stiffness between the groups (Mirbagheri et al. 2001). To determine reflex size, the average EMG activity was calculated for > 50 unperturbed steps and subtracted from the EMG activity of each of the perturbed steps. From the results, M1, M2, and M3 reflex sizes were calculated for each perturbed step, and average M1, M2, and M3 reflex sizes were calculated for each time bin of the step cycle.
Data analysis
To determine the degree to which each muscle’s EMG activity was modulated during walking, the modulation index was calculated in percent as: 100 × [(highest bin amplitude − lowest bin amplitude)/highest bin amplitude] (Zehr and Kido 2001; Kido et al. 2004a, b; Zehr and Loadman 2012; Makihara et al. 2014).
Reflex size was calculated in reference to each participant’s Mmax measured during natural standing. For example, the peak-to-peak H-reflex amplitude was normalized to the peak-to-peak Mmax amplitude, and the mean rectified M1 amplitude was normalized to the mean rectified Mmax amplitude. To compare the H-reflexes elicited at the same effective stimulus strength (i.e., the same M-wave size) across different phases of the step cycle, various stimulus intensities were used (Capaday and Stein 1986; Llewellyn et al. 1990; Edamura et al. 1991); and only the H-reflexes with comparable M-wave sizes were included in the analysis. Note that, in the present study, we did not elicit the Mmax at each part of the step cycle, to avoid potential negative effects of Mmax eliciting high intensity stimuli (e.g., discomfort and fatigue) altering locomotor EMG and kinematics. While the soleus Mmax amplitude could vary during walking (Simonsen and Dyhre-Poulsen 1999; Ferris et al. 2001), its range can remain close to that measured during natural standing (e.g., see Fig. 5 of Kido et al. 2004a). Thus, in this study, all the reflex size and M-wave size that accompanied the H-reflex were normalized to the Mmax during standing (Thompson et al. 2013a; Makihara et al. 2014). Typically, 10–15 responses were averaged in each of the 8 bins for both the H-reflex and stretch reflexes.
For comparing phase-dependent modulation of reflexes in two different groups, a two-way repeated measures ANOVA (groups × step cycle bins) was used, together with Student t test with Bonferroni correction as a post hoc test (i.e., for comparison between step cycle bins, α = 0.05, p < 0.0063 and α = 0.1, p < 0.0125). For simple comparison between participants without injuries and participants with SCI (e.g., age and Mmax), a Student t test was used.
In addition, for assessing the relationship between the H-reflex and stretch reflexes during walking, Pearson’s correlation coefficient (r value) was calculated between H-reflex size (in %Mmax) and stretch reflex size (in %Mmax) across the step cycle in each participant, and for each of the 8 step cycle bins across participants. We also performed correlation analysis on locomotor background (control) EMG (i.e., non-stimulated steps’ EMG of the corresponding time in the step cycle) and locomotor H-, M1, M2, and M3 reflex sizes.
To assess the impact of stretch or H-reflex activity on locomotor EMG in the subsequent step, soleus EMG was averaged for an extended post-stimulus (or perturbation) period that equaled the duration of the step cycle. Then, the number of clonus-like bursts that followed stretch or H-reflex (or reflex-eliciting stimulation/perturbation in the absence of reflex activity) was determined in each bin for each participant. Rhythmic bursts at 5–8 Hz [i.e., with 125–200 ms intervals (Latash et al. 1989; Rossi et al. 1990; Wallace et al. 2005; Gorassini et al. 2009; Wallace et al. 2012)] with peak amplitudes exceeding 1.5 × unstimulated step EMG amplitudes of the corresponding times in the step cycle were counted as clonic bursts.
Results
H -reflex and stretch reflexes during standing
Soleus H-reflex and stretch reflexes were measured, while the participant maintained a natural standing posture and kept soleus and TA EMG activity within the specified ranges. The absolute soleus background EMG amplitude was similar between participants without injuries and participants with incomplete SCI; 17 ± 2 (mean ± SE) μV for participants without injuries and 16 ± 2 μV for participants with SCI (p = 0.96, unpaired t test). When normalized to Mmax (i.e., mean rectified Mmax), it was 1.4 ± 0.2%Mmax for participants without injuries and 1.6 ± 0.3%Mmax for participants with SCI, and there was no significant difference (p = 0.56, unpaired t test). TA activity remained at the resting level, (i.e., < 7 μV) in both groups of participants. Thus, the background (pre-stimulus) EMG profiles during standing were very similar between these groups of participants.
Mmax and Hmax did not differ significantly between the groups. Peak-to-peak Mmax was 8.0 ± 1.6 (mean ± SE) mV in participants without injuries and 5.5 ± 0.7 mV in participants with SCI (p = 0.19), and the peak-to-peak Hmax was 3.2 ± 1.4 mV in participants without injuries and 2.8 ± 0.8 mV in participants with SCI (p = 0.83). The resulting Hmax/Mmax ratio was not significantly different (p = 0.33); 0.35 ± 0.06 for participants without injuries and 0.46 ± 0.08 for participants with SCI. Mean rectified Hmax was 32 ± 5 (mean ± SE)%Mmax in participants without injuries and 51 ± 13%Mmax in participants with SCI, not significantly different between the groups (p = 0.19).
For the soleus stretch reflexes, centers of responses (i.e., peak latencies) for the M1, M2, and M3 were similar between the groups: 56.8 ± 1.1 (mean ± SE) ms, 73.7 ± 1.0 ms, and 88.6 ± 0.3 ms for M1, M2, and M3 in participants without injuries; and 54.3 ± 0.5 ms, 72.1 ± 0.6 ms, and 88.8 ± 0.7 ms for M1, M2, and M3 in participants with SCI (p > 0.07 for M1, M2, and M3). In contrast, the stretch reflex sizes differed significantly between the groups. Mean rectified M1, M2, and M3 were 3.3 ± 0.6 (mean ± SE)%Mmax, 2.4 ± 0.4%Mmax, and 2.9 ± 0.6%Mmax in participants with SCI; they were larger than those in participants without injuries (1.7 ± 0.2%Mmax, 1.8 ± 0.3%Mmax, and 1.4 ± 0.3%Mmax for M1, M2, and M3, respectively) (p = 0.02 for M1, p = 0.31 for M2, and p = 0.03 for M3, unpaired t test). Comparisons of the peak-to-peak M1, M2, and M3 values between the groups showed similar trends, but did not yield significant differences (p > 0.26 for M1, M2, and M3): in participants with SCI vs. participants without injuries 2.9 ± 0.5 vs. 2.3 ± 0.3%Mmax for M1, 2.3 ± 0.4 vs. 2.6 ± 0.3%Mmax for M2, and 2.6 ± 0.4 vs. 1.9 ± 0.4%Mmax for M3.
H-reflex during walking
Typical examples of soleus H-reflexes during standing and walking are shown in Fig. 2. The top row displays H-reflexes in a participant without injuries: during standing with the pre-defined natural standing level of soleus EMG activity; during the mid-stance phase of walking (i.e., bin 3); and during the mid–late-swing phase of walking (i.e., bin 7). The M-waves that accompany these H-reflexes were all similar in size. As documented previously (Capaday and Stein 1986; Kido et al. 2004a; Makihara et al. 2012), the soleus H-reflex was dynamically and phase-dependently modulated during walking. The amplitude was high in the mid-stance phase (i.e., bin 3) when the muscle was active and contributing to functionally meaningful force generation, whereas in the mid–late-swing phase (i.e., bins 7 and 8), when the muscle was not active [and its contraction could be harmful (i.e., causing tripping)], the amplitude was at its minimum (Capaday and Stein 1986; Stein and Capaday 1988). In contrast, in a participant with chronic incomplete SCI (bottom row), the H-reflex was large in the mid–late swing (bins 7 and 8), during which the participant without injuries showed no H-reflex.
Examples of the soleus H-reflex during standing and walking in a participant without known neurological injuries (top) and in a participant with chronic incomplete SCI (bottom). Height, weight, and age are similar in these two individuals. More than 10 EMG sweeps were averaged for each panel. For each trace, the stimulus is indicated (with arrow) and the M-wave and H-reflex are labeled. In the participant without injuries, the soleus H-reflex was large in the mid-stance phase (i.e., bin 3) when the muscle was active and contributing to functionally meaningful force generation, and was completely suppressed in the mid–late-swing phase (i.e., bins 7 and 8) when the muscle was not active. In the participant with SCI, the H-reflex was large in the mid–late swing, in stark contrast to the normal subject
Soleus and TA EMG and H-reflex modulation over the step cycle (group mean ± SE) is shown in Fig. 3. We measured both peak-to-peak and mean rectified values for the H-reflex and stretch reflexes (next section). Since both modes of analysis yielded similar results, only mean rectified values are presented in here and in the sections below. Although the number of step cycle bins used in this study (i.e., 8) was smaller than in some previous studies of locomotor reflex modulation (Hase and Stein 1998; Zehr et al. 1998a, b; Kido et al. 2004b), it was sufficient to delineate phase-dependent modulation (as it did in Sinkjaer et al. 1996a, 1999; Zehr and Loadman 2012). In participants with SCI, soleus EMG during control (i.e., unperturbed) steps was less modulated than participants without injuries (p < 0.0001 by unpaired t test) (Fig. 3a, Table 3). Similarly, H-reflex modulation over the step cycle was less in participants with SCI than in participants without injuries (p = 0.002). The differences between the two groups in H-reflex amplitude and modulation was confirmed by a two-way (groups × step cycle bins) repeated measures ANOVA, which indicated significant effects of groups (p = 0.02), bins (p < 0.0001), and their interaction (p = 0.02). Notably, the H-reflex was larger in participants with SCI than in participants without injuries in bins 4–8 (i.e., the mid–late stance phase through the late-swing phase). In Fig. 3e, these differences (by Student t test with Bonferroni correction) are indicated by * (α = 0.05, p < 0.0063) and # (α = 0.1, p < 0.0125). In addition, in participants with SCI, the H-reflex in bin 8 (late-swing phase) was significantly larger than that in bin 6 (mid-swing phase) (Fig. 3e), indicating that H-reflex modulation was not only reduced but also was abnormal in form in the swing phase of the step cycle.
Soleus and TA EMG activity and H-reflexes, during walking in participants with chronic incomplete SCI (N = 9, mean ± SE) and participants without injuries (N = 9, mean ± SE). b, d, f Values for each individual with SCI. For calculating H-reflex size, for each participant, ≥ 10 EMG sweeps in each of eight equal bins of the step cycle were averaged together and normalized to Mmax. In general, bins 1–4 correspond to the stance phase, bin 5 to the stance-swing transition, and bins 6–8 to the swing phase. a, b Soleus EMG in control (unperturbed) steps. More than 100 control steps were averaged for each subject. c, d TA EMG amplitude in μV. e, f Soleus mean rectified H-reflex in % standing Mmax. Horizontal lines labeled with “S” in e indicate the average Hmax sizes during standing. For all panels, statistically significant differences between the groups and between the bins (in participants with SCI only) by Student t test with Bonferroni correction are indicated with * (α = 0.05, p < 0.0063) and # (α = 0.1, p < 0.0125)
TA EMG was modulated over the step cycle in both participants without injuries and participants with SCI (Table 3, Fig. 3c, d), and its modulation index did not differ between the groups.
Stretch reflexes during walking
Figure 4 shows typical examples of soleus and TA EMG and ankle joint motion during walking, and soleus stretch reflexes in bin 3 and 7 of the step cycle in participants with chronic incomplete SCI. The top panels show the average ankle joint motion around the time of perturbation in control (i.e., unperturbed) steps (solid line) and perturbed steps (dotted line). Panels in the second row show the averaged [perturbed–unperturbed] EMG sweeps. Thus, the excitation caused by the perturbation appears as additional EMG activity. Panels in the bottom half are for locomotor EMG activity in the soleus (black line) and TA (red line) and ankle joint motion over the entire step cycle, obtained by averaging > 50 control (i.e., unperturbed) steps. Locomotor EMG activity and ankle joint motion in these participants were clearly different from those in participants without injuries (see Fig. 1b). In the participant of Fig. 4a, there was a noticeable burst of activity in the soleus during the swing phase (note that in this participant the toe dragged throughout the swing phase and never came off the ground), while there was little TA activity. In bin 3, the absolute sizes of M1 and M2 were 33 μV and 28 μV, similar to those of participants without injuries (mean rectified M1 and M2 are 27 ± 11 μV and 26 ± 4 μV, respectively, in participants without injuries); but in bin 7, in which participants without injuries exhibit little stretch reflex activity (i.e., < 2.5 μV), both M1 and M2 were clearly larger (69 μV and 80 μV, respectively). In the participant of Fig. 4b, there was no dorsiflexion throughout the swing phase, despite the presence of some TA activity in the early–mid-swing phase. In the soleus, clonus was present in the early–mid-stance phase and disappeared in the swing phase. Similar to the observation in the participant of Fig. 4a, M1 and M2 in bin 3 were not remarkable; however, in bin 7, they were strikingly clear and large (42 μV and 119 μV, respectively) in the absence of ongoing soleus EMG activity.
Soleus stretch reflexes elicited by 6° dorsiflexion perturbation at 250°/s in bin 3 and 7 of the step cycle and soleus and TA EMG and ankle joint motion during walking in two representative individuals (a, b) with chronic incomplete SCI. In each individual, the top panel shows the average ankle joint motion around the time of perturbation in unperturbed (i.e., control) steps (solid line) and perturbed steps (dotted line). The second panel shows the averaged [perturbed − unperturbed] EMG response. Ten-to-fifteen EMG responses were averaged together. The third panel shows locomotor EMG activity in the soleus (black) and TA (red). The fourth panel shows the average ankle joint motion of unperturbed steps (averaged over > 50 control steps)
Soleus stretch reflex modulation over the step cycle (group mean ± SE) is shown in Fig. 5. In participants with SCI, M1 modulation was significantly less than in participants without injuries (p = 0.04, unpaired t test) (Fig. 5a, Table 3). Although the patterns differed from those in participants without injuries, M2 and M3 were modulated in participants with SCI; their extents of modulation were not significantly different from those of normal participants (p > 0.08 for both measures) (Table 3).
Soleus stretch reflexes during walking in participants with chronic incomplete SCI (blue in a, c, and e, N = 9, mean ± SE) and participants without injuries (black in a, c, and e, N = 9, mean ± SE). For each participant, ≥ 10 EMG responses in each of eight equal bins of the step cycle were averaged together and normalized to Mmax. In general, bins 1–4 correspond to the stance phase, bin 5 to the stance-swing transition, and bins 6–8 to the swing phase. M1 (a, b), M2 (c, d), and M3 (e, f) reflex sizes are calculated as mean rectified values. In a, c, and e, horizontal lines labeled with “S” indicate the average M1, M2, or M3 sizes during standing (the blue line is for the group with SCI, and the black line is for the group without injuries). b, d, f Values for each individual with SCI. For all panels, statistically significant differences between the groups and between the bins (in participants with SCI only) by Student t test with Bonferroni correction are indicated with * (α = 0.05, p < 0.0063) and # (α = 0.1, p < 0.0125)
The differences in stretch reflex modulation and reflex amplitudes between the groups were further evaluated by a two-way (groups × step cycle bins) repeated measures ANOVA. For M1, the effect of groups (p = 0.0025), bins (p = 0.0075), and their interaction (p = 0.0004) were all significant. The post hoc analysis (Student t test with Bonferroni Correction) indicated that, in bins 7 and 8 (i.e., mid–late-swing phase), M1 was significantly larger in participants with SCI than in participants without injuries and that in participants with SCI, M1 in the late-swing phase was larger than that in late stance (Fig. 5a). These results suggest that M1 modulation is not only reduced, but is also abnormal in participants with SCI. The effect of groups (p = 0.004), bins (p = 0.006), and their interaction (p = 0.0008) were all significant for M2 modulation. Post-hoc tests indicated that, in bins 5, 7, and 8, M2 was significantly larger in participants with SCI than in participants without injuries; and that, in participants with SCI, M2 was larger in the late-swing phase than in the late stance or early swing phase (Fig. 5c). The general features of M2 modulation in participants with SCI were similar to those of M1. During the stance phase, M2 was not notably different from that in participants without injuries. However, in the mid–late-swing phase, the difference between the groups became very clear: M2 was suppressed in participants without injuries and enhanced in participants with SCI. For M3, the effect of bins was significant (p = 0.004), as expected from the modulation index analysis above. However, the effect of groups and the groups × bins interaction were not significant (p = 0.49 and 0.35, respectively). Overall, the modulation of M3 was not markedly different between participants with SCI and participants without injuries (Fig. 5e). In Fig. 5, differences detected by Student t test with Bonferroni Correction are indicated by * (α = 0.05, p < 0.0063) and # (α = 0.1, p < 0.0125).
Relations between background EMG, H-reflex and stretch reflexes during walking
To estimate how much of the variance in reflex size would be explained by locomotor background EMG activity, the correlation between background EMG (control EMG of non-stimulated steps for the corresponding time in the step cycle) and H, M1, M2, and M3 reflexes were assessed in each participant. As previously shown (e.g., Capaday and Stein 1986), in neurologically normal participants, the size of the locomotor H-reflex was strongly correlated with background EMG activity [r = 0.91 ± 0.02 (group mean ± SE)], and the r2 value of 0.84 ± 0.04 indicates that a large portion of reflex size variation is explicable by background EMG activity. M1, M2, and M3 reflex sizes also correlated with background EMG although to a lesser extent (r = 0.70 ± 0.07, 0.81 ± 0.04, and 0.48 ± 0.16, respectively), and their variations were partially explainable by background EMG activity (r2 = 0.52 ± 0.08, 0.66 ± 0.07, and 0.44 ± 0.11, respectively). In participants with SCI, these relations were far weaker for both H and stretch reflexes; (r2 = 0.23 ± 0.05, 0.07 ± 0.02, 0.11 ± 0.03, and 0.26 ± 0.09, for H, M1, M2, and M3, respectively). The coefficient of determination (i.e., r2) differed significantly between the groups of participants with and without SCI for the H-reflex (p < 0.0001, by two-tailed t test), M1 (p = 0.0008), and M2 (p < 0.0001), but not for M3 (p = 0.25). Figure 6a, b exemplifies these relations in representative individuals of both groups.
Correlation between the soleus locomotor background EMG and H-reflex (a) or M1 stretch reflex size (b) and correlation between H-reflex size and M1, M2, or M3 stretch reflex size (c). a Mean rectified H-reflex sizes from 8 bins of the step cycle are plotted against background EMG (i.e., control EMG of non-stimulated steps for the period corresponding to the time of reflex elicitation) in a representative participant with SCI (blue crosses) and a participant without SCI (black circles). Coefficient of determination r2 values and the corresponding regression lines are also indicated in blue (participant with SCI) and black (participant without SCI). b Mean rectified M1 reflex sizes from eight bins of the step cycle are plotted against background (control) EMG in the same representative individuals as in a. c Pearson’s correlation coefficient r value is calculated for each of the 8 bins of the step cycle for each group. Light red bands indicate statistically significant (p < 0.05) r value ranges
To assess whether there is a predictable relationship between the sizes of H-reflex and stretch reflexes in the soleus, we calculated Pearson’s correlation coefficient (r value) across the step cycle in each participant. In participants without SCI, a moderate positive correlation was found for all three components of the stretch reflex; r = 0.62 ± 0.07 (group mean ± SE) for H × M1, r = 0.73 ± 0.05 for H × M2, and r = 0.50 ± 0.16 for H × M3. In contrast, in participants with SCI, the correlation was weak to none for all three components of the stretch reflex; r = 0.03 ± 0.12 for H × M1, r = 0.23 ± 0.12 for H × M2, and r = 0.24 ± 0.11 for H × M3. We also calculated the r value for each of the eight-step cycle bins across participants. Figure 6c summarizes the correlation coefficients between H-reflex size and M1, M2, or M3 stretch reflex size. In general, there were no obvious trends in the r value distribution for any of the three stretch reflex components in either group.
Effects of reflex elicitation in the late-swing phase of walking
As summarized in Fig. 3c, tibial nerve stimulation in the mid–late-swing phase (i.e., bins 7 and 8) elicited little or no soleus H-reflex in participants without injuries. Similarly, dorsiflexion perturbations applied in the mid–late-swing phase elicited little or no stretch reflexes in participants without injuries (Fig. 5). In contrast, in participants with SCI, H-reflex and stretch reflexes were present in this phase, and were often larger than those in the mid–late stance phase (Figs. 3c, 5). To assess the impact of this abnormal reflex activity in participants with SCI, soleus EMG activity was averaged for an extended post-stimulus (or perturbation) period that equaled the duration of the step cycle. Figure 7 shows a typical example of post-stretch reflex EMG activity after a perturbation in bin 8. The averaged perturbed EMG (N = 15, solid) is shown with the average unperturbed EMG (N = 62, dotted). In this example, the perturbation was introduced at ≈ 1350 ms after foot contact (i.e., late-swing phase). It elicited large stretch reflexes and clonic EMG bursts over the subsequent stance phase; clonus was so robust that it replaced the fused EMG activity that was present during unperturbed stance. Similar induction and/or amplification of clonic bursts in the immediately following step were observed after perturbations in bin 8 in all 9 participants with SCI studied here (Fig. 9a). In clear contrast, in participants without injuries, elicitation of stretch reflexes or reflex-eliciting dorsiflexion perturbations rarely triggered any discernable clonic bursts across the step cycle (Fig. 9c).
Soleus EMG activity after 7° dorsiflexion perturbation at 240°/s in bin 8 in a participant with SCI. Fifteen perturbed EMG sweeps were averaged together (solid), and 62 unperturbed EMG sweeps over the corresponding phase of step cycle were averaged together (dotted). A black arrow with a dashed line indicates the onset of perturbation. A blue arrow (around 1500 ms) indicates the foot contact of the step immediately after. Perturbation introduced at ≈ 1350 ms after foot contact (i.e., ≈ 150 ms before the following foot contact) elicited large stretch reflexes (M1 and M2 appear to be fused in this figure, since the horizontal scale displays > 1000 ms after perturbation) and clonic EMG bursts over the subsequent stance phase. Robust clonus replaced the fused mid–late stance EMG activity that was present in unperturbed steps
Figure 8 shows the EMG activity after tibial nerve stimulation in 8 bins of the step cycle in a typical participant with SCI. Eleven-to-fifteen EMG sweeps are averaged for each bin. It is clear that H-reflex elicitation in bins 4, 5, and 6 (i.e., mid–late stance phase and early swing phase) did not induce clonus, whereas in bins 8 (late swing) and 1 (early stance), it triggered clonic bursts. Interestingly, this induction of clonus or clonus-like phasic EMG bursts [of 5–8 Hz, (Latash et al. 1989; Rossi et al. 1990; Wallace et al. 2005; Gorassini et al. 2009; Wallace et al. 2012)] by H-reflex elicitation was a phase-dependent phenomenon. The number of clonic bursts after H-reflex elicitation progressively declined from bin 8 to bin 3; clonus appeared to cease in the late stance phase, regardless of how many bursts had been produced up to that point. Across all SCI participants of this study, we observed that H-reflex-eliciting tibial nerve stimulation in bin 8 triggered clonus or clonus-like burst(s) of rhythmic excitation, whereas the same stimulation in bins 4 and 5, despite producing larger H-reflexes, did not trigger similar rhythmic bursts (Fig. 9b). In participants without injuries, elicitation of the H-reflex or the same relative intensity of tibial nerve stimulation that elicited the H-reflex in the stance phase rarely triggered any discernable clonic bursts across the step cycle (Fig. 9d). Exploration of the physiological mechanisms underlying these observations was beyond the scope of this study. Further studies are needed to understand the mechanisms of clonus and its susceptibility to spinal and supraspinal inhibition in people with chronic SCI.
Soleus EMG activity after H-reflex-eliciting tibial nerve stimulation in 8 bins of the step cycle in a participant with SCI. In each panel, 11–15 EMG sweeps following the H-reflexes with similar M-wave sizes were averaged together. Black arrow heads indicate the times of foot contact in the steps immediately after stimulation. H-reflex elicitation in bins 8 (late swing) and bins 1 and 2 (early and mid-stance) led to several clonic bursts that replaced the fused mid–late stance push-off burst
Counts of clonus-like rhythmic bursts in the locomotor soleus EMG activity after dorsiflexion perturbation (a, c) or H-reflex-eliciting tibial nerve stimulation (b, d) in 8 bins of the step cycle in participants with SCI (a, b) and in participants without injuries (c, d). Each color represents a single participant. In participants with SCI, dorsiflexion perturbation or H-reflex-eliciting tibial nerve stimulation often triggered clonus-like (i.e., 5–8 Hz burst) in bins 7, 8, 1, and 2. In contrast, such phasic bursts were rare across participants without injuries at any phases of the step cycle
Discussion
This study examined soleus H-reflexes and stretch reflexes during standing and walking in people with spasticity due to chronic incomplete SCI. During standing, the differences in sizes of soleus H-reflex and stretch reflexes between participants with and without SCI were small and often statistically insignificant. Such insignificant differences were surprising, as higher Hmax/Mmax ratios and exaggerated stretch reflexes have been reported in spastic participants with SCI and other CNS disorders previously (Taylor et al. 1984; Brown 1994; Thompson et al. 2009b). This could be partly explained by baclofen [which increases the stretch reflex threshold in people with spasticity due to multiple sclerosis (Nielsen et al. 2000; Nielsen and Sinkjaer 2000)] and/or by other anti-spastic medications that our participants with SCI were taking. In addition, several inhibitory mechanisms acting on these spinal reflexes would be reduced in actively contracting muscles in participants with and without spasticity (Nielsen et al. 2005; Dietz and Sinkjaer 2012, 2015), and thus, might have diminished the differences between participants without injuries and participants with SCI.
Nevertheless, modulation of the M1 and M2 stretch reflexes and H-reflex was clearly abnormal during walking in participants with SCI. Normal positive correlation between locomotor background EMG and reflex excitability was rarely found in those individuals. An important implication of these observations is that reflexes elicited in selected passive states, during isolated joint movements, and/or during static motor tasks do not necessarily indicate how the reflexes function or malfunction during specific phases of dynamic motion, such as walking. Here, we found that the excitability of spinal stretch reflex pathways was abnormally high from the stance-swing transition through the late-swing phase in ambulatory individuals with chronic incomplete SCI.
H-reflex and stretch reflex modulation during walking in people with chronic incomplete SCI
For stretch reflexes, group Ia and II muscle spindle afferents are excited by stretch of the nerve endings in the spindle, affected by γ-motoneuron mediated fusimotor control, and muscle stretch (tension change) excites group Ib Golgi tendon organ afferents; for the H-reflex, group Ia (and some II) muscle spindle afferent axons and group Ib Golgi tendon organ afferent axons are excited electrically (Magladery et al. 1951; Lundberg et al. 1978; Henneman and Mendell 1980; Jankowska and McCrea 1983; McKeon and Burke 1983; Morita et al. 1998; Schafer et al. 1999; Duysens et al. 2000; Jankowska and Edgley 2010; Vincent et al. 2017; Nichols 2018). While some differences between stretch and H-reflexes were noted as in earlier studies (Morita et al. 1998; Andersen and Sinkjaer 1999), the present study found that the spinal short-latency M1 stretch reflex and the H-reflex (both thought to originate mainly from Ia afferents) were both suppressed in the swing phase in participants without injuries but not in participants with SCI (Figs. 3c, 5). This may suggest reduced inhibition of Ia excitatory pathways in this phase in participants with SCI. Modulation of the spinal medium-latency M2 reflex, presumably mainly group II afferent-mediated (Corna et al. 1995; Schieppati and Nardone 1997; Nardone and Schieppati 1998; Grey et al. 2001; Af Klint et al. 2010), was similar to that of M1 (for both participants with and without SCI), with strikingly large amplitudes in the mid–late-swing phase for participants with SCI (Fig. 5). Exaggerated M2 responses in these participants could be due to reduced inhibition of spindle afferent II excitation, increased oligo or polysynaptic Ia excitation, and/or increased excitation of other afferents. Actions of excitatory and inhibitory interneurons that receive input from Ib afferents could also affect M2 modulation (Schafer et al. 1999; Jankowska and Edgley 2010). Since our current understanding of Ia, II, and Ib afferent firing patterns and functional contributions of those afferents to muscle activity is largely based on feline studies (e.g., (Jankowska 1979; Jankowska and McCrea 1983; Donelan and Pearson 2004; Jankowska and Edgley 2010; Hatz et al. 2012), it is not clear in humans how those afferents from plantarflexors fire during locomotion and whether and how their input to interneurons result in muscle activation. However, presuming fundamental similarities in the characteristics of those afferents across species, it is possible that spindle afferent II could be firing in the late-swing phase (i.e., pre-stance) (Donelan and Pearson 2004; Hatz et al. 2012) even when there is no EMG activity, and it is likely that Ib and II afferents would be firing when there is EMG activity (Donelan and Pearson 2004; Hatz et al. 2012). These afferent firing behaviors, together with altered modulation or excitability of Ib/II interneurons, may explain abnormal swing phase burst in the soleus EMG (e.g., Fig. 4a) or abnormally large M2 response in the late-swing phase (e.g., Fig. 4b) in pathologic gait, at least partly. Clearly, further studies are needed to determine which afferents (or afferent combinations) contribute to M2 in individuals with SCI.
The long-latency M3 response, which may be a transcortical or subcortical response (Sinkjaer et al. 1999; Christensen et al. 2001; Zuur 2013), was phase-dependently modulated similarly in both groups of participants. Although currently, there is no direct evidence indicating the pathway(s) of M3 in participants with SCI, a surprisingly near normal pattern of M3 modulation (in contrast to abnormal M1 and M2 modulation) and the presence of M3 in the mid–late stance phase of walking in some participants with incomplete SCI (e.g., Fig. 4b) (in whom some corticospinal connections could remain), supports the possibility that M3 is a transcortical response in these participants.
One of the important analyses made in this study is the H-reflex—stretch reflex correlation analysis. As mentioned in “Introduction”, although the H-reflex is often used to investigate Ia excitation of homonymous motoneurons (Schieppati 1987; Zehr 2002; Misiaszek 2003), H-reflex and M1 stretch reflex do not necessarily respond to modulatory input in the same way (Nielsen et al. 1994; Sinkjaer et al. 1996a; Morita et al. 1998). In the present study, stretch reflexes and H-reflex were measured in the same individuals during the same task of treadmill walking, which provided a unique opportunity for assessing the predictability of stretch reflex excitability from the H-reflex measurement. In individual participants without SCI, moderate positive correlation was found between the H-reflex and all three components of stretch reflexes across the 8 equal bins of the step cycle, supporting a moderate predictability of stretch reflex behavior from the H-reflex measurements during walking in neurologically normal individuals. In contrast, in participants with SCI, such correlation was weak to non-existing, suggesting that the locomotor H-reflex amplitudes would not provide reliable estimates of stretch reflex excitability in individuals of this population. Furthermore, across individuals, H-reflex size at a given phase of step cycle did not necessarily predict M1 (or M2 or M3) reflex size effectively; as shown in Fig. 6c, H-reflex size did not systematically correlate with M1, M2, or M3 stretch reflex size in any of the eight step cycle bins (with potential exceptions in the mid-to-late-swing phase in the group without SCI, in whom the reflexes in these bins are small to begin with (see Figs. 3c, 5). Thus, the present observation suggests that investigation of the H-reflex would not substitute for investigation of M1, M2, or M3 stretch reflexes in the soleus, especially in individuals with chronic SCI.
Etiology of abnormal locomotor reflex modulation in people with incomplete SCI
In individuals without known neurological injuries, the soleus H-reflex is task- and phase-dependently modulated during walking. Task-dependent reduction of reflex gain from standing to walking to running is thought to be due to increased presynaptic inhibition (Capaday and Stein 1987b; Stein and Capaday 1988) caused by supraspinal (including corticospinal) control (Hodapp et al. 2007a, b). Phase-dependent modulation of the H-reflex is likely to be generated by presynaptic inhibition (Edamura et al. 1991; Yang and Whelan 1993), within the spinal cord or below the brainstem mechanisms (Hodapp et al. 2007b). Larger locomotor H-reflex amplitude in general and abnormal patterns of H-reflex and M1 and M2 stretch reflex modulation are consistent with the expected effects of impaired presynaptic inhibition of these spinal reflex pathways, of both spinal and supraspinal origin.
In addition, it is probable that other inhibitory mechanisms are also impaired, further exacerbating problems due to abnormal stretch reflexes (Taylor et al. 1984; Mailis and Ashby 1990) and changes in spinal motoneurons and interneurons (e.g., persistent inward current) (Hultborn 2003; Gorassini et al. 2004; Hornby et al. 2006; Heckman et al. 2008). Reduced corticospinal activation of the TA that results in weak dorsiflexion and foot drop (Knutsson 1981; Yang et al. 1991; Davey et al. 1999; Barthelemy et al. 2010) would also reduce reciprocal inhibition of the soleus (Boorman et al. 1996) (see also Willerslev-Olsen et al. 2014) even if reciprocal inhibition itself is normal. In participants with SCI, reciprocal inhibition between the plantarflexors (e.g., soleus) and dorsiflexors is often abnormal (Ashby and Wiens 1989; Boorman et al. 1991; Crone et al. 2003; Thompson et al. 2009b; Knikou and Mummidisetty 2011), and would further reduce the suppression of the soleus motoneuron excitability in the stance-swing transition through the late-swing phase. Since TA was active during the swing phase in the (majority of) present participants with incomplete SCI (see Fig. 3b), it is possible that impaired reciprocal inhibition contributed to ineffective suppression of soleus and its stretch reflexes during the swing phase. Ib inhibition (Morita et al. 2006), recurrent inhibition (Shefner et al. 1992), and cutaneous reflexes (Jones and Yang 1994) are also altered after SCI, and these pathways may interact with each other (Bastiaanse et al. 2006; Knikou 2007; Knikou et al. 2007; Nakajima et al. 2014), contributing to spastic locomotor EMG patterns and impaired stretch reflex modulation. Thus, it is probable that multiple inhibitory mechanisms are depressed during walking, resulting in disorganized and ineffective activation of multiple muscles in participants with SCI.
Functional implications
Normally, the soleus H-reflex and all three stretch reflex components are phase-dependently modulated during walking, in accord with soleus EMG activity, which is high in the mid-stance phase and very low (little-to-none) in the swing phase (Capaday and Stein 1986; Sinkjaer et al. 1996a, 1999; Andersen and Sinkjaer 1999). The present results showed that, in individuals with SCI, modulation of soleus EMG activity, H-reflex, and spinal M1 and M2 stretch reflexes was reduced during walking and was abnormal in form. Soleus EMG was unsuppressed during the swing phase but reduced in mid-stance (Fig. 3a). M1 and M2 were not only unsuppressed during the swing phase, but also often larger in the mid–late-swing phase, which may be explained in part by inadequately hyperactive soleus elevating the reflex excitability. Inversely, enhanced and unsuppressed spinal stretch reflex pathways might contribute to the reduced soleus suppression in the swing phase. These possibilities require further examinations in the future.
In this study, we observed that stretch reflex elicitation in the mid–late-swing phase induces clonus that continues into the stance phase, which would, in turn, interfere with smooth forward motion (Nielsen et al. 1998). Unexpected rapid (stumble-like) stretches used in this study would induce afferent excitation that is faster and more synchronous than that occurring during normal unperturbed walking. However, considering that many individuals with SCI experience toe drag and tripping (i.e., perturbation during the swing phase) often enough during their daily locomotion, it is possible that something similar to what was observed in this study, or of lesser extent, would be experienced by ambulatory individuals with chronic incomplete SCI (it is not unusual to witness individuals with SCI getting stuck in the middle of a step, not being able to propel forward due to toe catching or clonus, during overground walking without stimulation or perturbations, in the laboratory or in the community). Large bursts of clonic activity in the early and early–mid-stance phase enhance generation of posterior breaking force (Turns et al. 2007), reducing forward propulsion. Furthermore, these bursts are often followed by more clonic bursts and/or a much reduced push-off burst in the mid–late stance phase (e.g., Fig. 7), which would reduce propulsive force generation (Turns et al. 2007) and gait speed (Bowden et al. 2006). Thus, individuals with exaggerated spinal stretch reflexes, like those in this study, may encounter these gait obstructions, in response to small extra ankle rotations throughout their daily locomotion.
Together with the present findings, frequent occurrence of clonus in individuals with chronic SCI (e.g., Latash et al. 1989; Rossi et al. 1990; Boyraz et al. 2009, Figs. 7, 8) and presumed contribution of group Ia and II afferents to clonus (Boyraz et al. 2009; Uysal et al. 2009, 2011) urge further investigation of stretch reflexes and its relation to clonus during dynamic motion. To better understand afferent contributions to unperturbed spastic gait, our future plans include stretch reflex examinations using slow small stretches (e.g., Mazzaro et al. 2005, 2007; Willerslev-Olsen et al. 2014) and/or “unloading” perturbations (Sinkjaer et al. 2000).
Consideration of methodological limitations
Several methodological limitations in the present study would deserve brief discussion. First, in the present study, we did not measure the soleus Mmax across the different phases of walking. Since the soleus Mmax size varies across different ankle angles (Frigon et al. 2007) and across different phases of step cycle (Simonsen and Dyhre-Poulsen 1999; Ferris et al. 2001) with different patterns and extents in different individuals (Simonsen and Dyhre-Poulsen 1999), the locomotor reflex modulation presented here (Fig. 3) may not be completely accurate. Since the locomotor Mmax across the step cycle could remain close to Mmax during natural standing (as shown in Fig. 5 of Kido et al. 2004a), in the present study, all reflex sizes were normalized to the size of soleus Mmax during standing, instead of the locomotor Mmax of a corresponding phase of step cycle. This limitation in normalization is a trade-off to avoiding the potential detrimental effects of making the Mmax measurements on reflex measurements during walking. To find the true Mmax at each bin of the step cycle, a large number of additional stimuli and an even larger number of steps would be required; both of which could result in fatigue and potential alterations of gait in individuals with SCI. While acknowledging this limitation in our data set, we are confident that the reflex and EMG modulation patterns captured in this study reflect existing abnormalities in these individuals with SCI. Clear M1, M2, and H-reflexes seen in EMG (e.g., Figs. 2, 4) in the late-swing phase, for example, are not the products of normalization.
Second, in the present study, the majority of participants with SCI had been on a stable dose of baclofen and/or other anti-spasticity medication (e.g., tizanidine) at the time of experiment. Since those drugs can affect spinal reflex characteristics (Bes et al. 1988; Latash et al. 1989; Corna et al. 1995; Nielsen et al. 2000; Nielsen and Sinkjaer 2000; Maupas et al. 2004; Mirbagheri et al. 2010; Uysal et al. 2011; Mirbagheri 2015), it is probable that the reflex amplitudes and modulation reported here were under influence of such medication. In investigating the locomotor stretch reflex modulation in individuals with chronic incomplete SCI, our aim was to elucidate abnormal reflex activity during their “usual” gait, which had been established over many months to years with or without medication. In individuals who have been on stable doses of medication, temporal removal of medication would disturb not only their physiological normalcy but also their established, not necessarily normal but functional gait. Thus, while acknowledging medication-related limitations in interpretation, we would value the present participants’ data, as they represent (at least partly) a large proportion of individuals with chronic SCI who live their lives with stable anti-spasticity medication and remaining spasticity.
Conclusion
This study showed for the first time that the short and medium latencies soleus stretch reflexes were abnormally large in the mid–late-swing phase in ambulatory individuals with chronic incomplete SCI. It is possible that multiple spinal and supraspinal pathways that contribute to other CNS populations’ spastic movement disorders cumulatively and/or through interactions among themselves (Dietz and Sinkjaer 2007; Nielsen et al. 2007; Burke et al. 2013; Li and Francisco 2015) could also contribute to the abnormal locomotor reflex modulation observed in the present participants with SCI. The present observations support a possibility that hyperactive spinal stretch reflexes in the mid–late-swing phase can affect the following stance phase. Studies including full measurements of lower extremity kinematics will be necessary in the future, to examine such potential impacts of abnormal stretch reflexes on locomotion in people with chronic incomplete SCI.
References
Adams MM, Hicks AL (2005) Spasticity after spinal cord injury. Spinal Cord 43:577–586. https://doi.org/10.1038/sj.sc.3101757
Af Klint R, Mazzaro N, Nielsen JB, Sinkjaer T, Grey MJ (2010) Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J Neurophysiol 103:2747–2756. https://doi.org/10.1152/jn.00547.2009
Andersen JB, Sinkjaer T (1995) An actuator system for investigating electrophysiological and biomechanical features around the human ankle joint during gait. IEEE Trans Rehabil Eng 3:299–306
Andersen JB, Sinkjaer T (1999) The stretch reflex and H-reflex of the human soleus muscle during walking. Mot Control 3:151–157
Ashby P, Wiens M (1989) Reciprocal inhibition following lesions of the spinal cord in man. J Physiol 414:145–157
Barbeau H, Ladouceur M, Mirbagheri MM, Kearney RE (2002) The effect of locomotor training combined with functional electrical stimulation in chronic spinal cord injured subjects: walking and reflex studies. Brain Res Brain Res Rev 40:274–291
Barthelemy D, Willerslev-Olsen M, Lundell H, Conway BA, Knudsen H, Biering-Sorensen F, Nielsen JB (2010) Impaired transmission in the corticospinal tract and gait disability in spinal cord injured persons. J Neurophysiol 104:1167–1176. https://doi.org/10.1152/jn.00382.2010
Bastiaanse CM, Degen S, Baken BC, Dietz V, Duysens J (2006) Suppression of cutaneous reflexes by a conditioning pulse during human walking. Exp Brain Res 172:67–76
Bes A, Eyssette M, Pierrot-Deseilligny E, Rohmer F, Warter JM (1988) A multi-centre, double-blind trial of tizanidine, a new antispastic agent, in spasticity associated with hemiplegia. Curr Med Res Opin 10:709–718. https://doi.org/10.1185/03007998809111122
Boorman G, Hulliger M, Lee RG, Tako K, Tanaka R (1991) Reciprocal Ia inhibition in patients with spinal spasticity. Neurosci Lett 127:57–60
Boorman GI, Lee RG, Becker WJ, Windhorst UR (1996) Impaired “natural reciprocal inhibition” in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr Clin Neurophysiol 101:84–92
Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA (2006) Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke 37:872–876. https://doi.org/10.1161/01.STR.0000204063.75779.8d
Boyraz I, Oktay F, Celik C, Akyuz M, Uysal H (2009) Effect of cold application and tizanidine on clonus: clinical and electrophysiological assessment. J Spinal Cord Med 32:132–139
Brown P (1994) Pathophysiology of spasticity. J Neurol Neurosurg Psychiatry 57:773–777
Burke D, Wissel J, Donnan GA (2013) Pathophysiology of spasticity in stroke. Neurology 80:S20–S26. https://doi.org/10.1212/WNL.0b013e31827624a7
Capaday C, Stein RB (1986) Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci 6:1308–1313
Capaday C, Stein RB (1987a) Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol 392:513–522
Capaday C, Stein RB (1987b) A method for simulating the reflex output of a motoneuron pool. J Neurosci Methods 21:91–104
Christensen LO, Petersen N, Andersen JB, Sinkjaer T, Nielsen JB (2000) Evidence for transcortical reflex pathways in the lower limb of man. Prog Neurobiol 62:251–272
Christensen LO, Andersen JB, Sinkjaer T, Nielsen J (2001) Transcranial magnetic stimulation and stretch reflexes in the tibialis anterior muscle during human walking. J Physiol 531:545–557
Corcos DM, Gottlieb GL, Penn RD, Myklebust B, Agarwal GC (1986) Movement deficits caused by hyperexcitable stretch reflexes in spastic humans. Brain 109(Pt 5):1043–1058
Corna S, Grasso M, Nardone A, Schieppati M (1995) Selective depression of medium-latency leg and foot muscle responses to stretch by an alpha 2-agonist in humans. J Physiol 484(Pt 3):803–809
Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB (2003) Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126:495–507
Davey NJ, Smith HC, Savic G, Maskill DW, Ellaway PH, Frankel HL (1999) Comparison of input-output patterns in the corticospinal system of normal subjects and incomplete spinal cord injured patients. Exp Brain Res 127:382–390
Decq P (2003) Pathophysiology of spasticity. Neurochirurgie 49:163–184
Dietz V, Sinkjaer T (2007) Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol 6:725–733. https://doi.org/10.1016/S1474-4422(07)70193-X
Dietz V, Sinkjaer T (2012) Spasticity. Handb Clin Neurol 109:197–211. https://doi.org/10.1016/B978-0-444-52137-8.00012-7
Dietz V, Sinkjaer T (2015) Secondary changes after damage of the central nervous system: significance of spastic muscle tone in rehabilitation. In: Dietz V, Ward N (eds) Oxford textbook of neurorehabilitation. Oxford University Press, Oxford
Donelan JM, Pearson KG (2004) Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol 92:2093–2104
Duysens J, Trippel M, Horstmann GA, Dietz V (1990) Gating and reversal of reflexes in ankle muscles during human walking. Exp Brain Res 82:351–358
Duysens J, Clarac F, Cruse H (2000) Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80:83–133
Duysens J, Bastiaanse CM, Smits-Engelsman BC, Dietz V (2004) Gait acts as a gate for reflexes from the foot. Can J Physiol Pharmacol 82:715–722
Edamura M, Yang JF, Stein RB (1991) Factors that determine the magnitude and time course of human H-reflexes in locomotion. J Neurosci 11:420–427
Ethier C, Imbeault MA, Ung V, Capaday C (2003) On the soleus H-reflex modulation pattern during walking. Exp Brain Res 151:420–425
Faist M, Ertel M, Berger W, Dietz V (1999) Impaired modulation of quadriceps tendon jerk reflex during spastic gait: differences between spinal and cerebral lesions. Brain 122(Pt 3):567–579
Ferris DP, Aagaard P, Simonsen EB, Farley CT, Dyhre-Poulsen P (2001) Soleus H-reflex gain in humans walking and running under simulated reduced gravity. J Physiol 530:167–180
Frigon A, Carroll TJ, Jones KE, Zehr EP, Collins DF (2007) Ankle position and voluntary contraction alter maximal M waves in soleus and tibialis anterior. Muscle Nerve 35:756–766
Fung J, Barbeau H (1989) A dynamic EMG profile index to quantify muscular activation disorder in spastic paretic gait. Electroencephalogr Clin Neurophysiol 73:233–244
Fung J, Barbeau H (1994) Effects of conditioning cutaneomuscular stimulation on the soleus H-reflex in normal and spastic paretic subjects during walking and standing. J Neurophysiol 72:2090–2104
Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF (2004) Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127:2247–2258. https://doi.org/10.1093/brain/awh243
Gorassini MA, Norton JA, Nevett-Duchcherer J, Roy FD, Yang JF (2009) Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J Neurophysiol 101:969–979
Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T (2001) Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol 534:925–933
Grey MJ, van Doornik J, Sinkjaer T (2002) Plantar flexor stretch reflex responses to whole body loading/unloading during human walking. Eur J Neurosci 16:2001–2007
Grey MJ, Nielsen JB, Mazzaro N, Sinkjaer T (2007) Positive force feedback in human walking. J Physiol 581:99–105. https://doi.org/10.1113/jphysiol.2007.130088
Hase K, Stein RB (1998) Analysis of rapid stopping during human walking. J Neurophysiol 80:255–261
Hatz K, Mombaur K, Donelan JM (2012) Control of ankle extensor muscle activity in walking cats. J Neurophysiol 108:2785–2793. https://doi.org/10.1152/jn.00944.2011
Heckman CJ, Johnson M, Mottram C, Schuster J (2008) Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14:264–275. https://doi.org/10.1177/1073858408314986
Henneman E, Mendell LM (1980) Functional organization of motoneuron pool and its inputs. In: Brookhart JM, Mountcastle VB (eds) Handbook of physiology: section 1: the nervous system, vol II. Part 1 &2: Motor control. American Physiological Society, Bethesda, pp 423–507
Hidler JM, Rymer WZ (1999) A simulation study of reflex instability in spasticity: origins of clonus. IEEE Trans Rehabil Eng 7:327–340
Hiersemenzel LP, Curt A, Dietz V (2000) From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology 54:1574–1582
Hodapp M, Klisch C, Berger W, Mall V, Faist M (2007a) Modulation of soleus H-reflexes during gait in healthy children. Exp Brain Res 178:252–260. https://doi.org/10.1007/s00221-006-0730-1
Hodapp M, Klisch C, Mall V, Vry J, Berger W, Faist M (2007b) Modulation of soleus H-reflexes during gait in children with cerebral palsy. J Neurophysiol 98:3263–3268
Hornby TG, Kahn JH, Wu M, Schmit BD (2006) Temporal facilitation of spastic stretch reflexes following human spinal cord injury. J Physiol 571:593–604. https://doi.org/10.1113/jphysiol.2005.102046
Hultborn H (2003) Changes in neuronal properties and spinal reflexes during development of spasticity following spinal cord lesions and stroke: studies in animal models and patients. J Rehabil Med (41 Suppl):46–55
Jankowska E (1979) New observations on neuronal organization of reflexes from tendon organ afferents and their relation to reflexes evoked from muscle spindle afferents. Prog Brain Res 50:29–36
Jankowska E, Edgley SA (2010) Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci 32:881–893. https://doi.org/10.1111/j.1460-9568.2010.07354.x
Jankowska E, McCrea DA (1983) Shared reflex pathways from Ib tendon organ afferents and Ia muscle spindle afferents in the cat. J Physiol 338:99–111. https://doi.org/10.1113/jphysiol.1983.sp014663
Jones CA, Yang JF (1994) Reflex behavior during walking in incomplete spinal-cord-injured subjects. Exp Neurol 128:239–248
Kawashima N, Yano H, Ohta Y, Nakazawa K (2006) Stretch reflex modulation during imposed static and dynamic hip movements in standing humans. Exp Brain Res 174:342–350. https://doi.org/10.1007/s00221-006-0470-2
Kido A, Tanaka N, Stein RB (2004a) Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82:238–248
Kido A, Tanaka N, Stein RB (2004b) Spinal reciprocal inhibition in human locomotion. J Appl Physiol 96:1969–1977
Knikou M (2007) Plantar cutaneous input modulates differently spinal reflexes in subjects with intact and injured spinal cord. Spinal Cord 45:69–77. https://doi.org/10.1038/sj.sc.3101917
Knikou M (2013) Functional reorganization of soleus H-reflex modulation during stepping after robotic-assisted step training in people with complete and incomplete spinal cord injury. Exp Brain Res 228:279–296. https://doi.org/10.1007/s00221-013-3560-y
Knikou M, Mummidisetty CK (2011) Reduced reciprocal inhibition during assisted stepping in human spinal cord injury. Exp Neurol 231:104–112. https://doi.org/10.1016/j.expneurol.2011.05.021
Knikou M, Kay E, Schmit BD (2007) Parallel facilitatory reflex pathways from the foot and hip to flexors and extensors in the injured human spinal cord. Exp Neurol 206:146–158
Knikou M, Angeli CA, Ferreira CK, Harkema SJ (2009a) Soleus H-reflex gain, threshold, and amplitude as function of body posture and load in spinal cord intact and injured subjects. Int J Neurosci 119:2056–2073
Knikou M, Angeli CA, Ferreira CK, Harkema SJ (2009b) Soleus H-reflex modulation during body weight support treadmill walking in spinal cord intact and injured subjects. Exp Brain Res 193:397–407. https://doi.org/10.1007/s00221-008-1636-x
Knutsson E (1981) Gait control in hemiparesis. Scand J Rehabil Med 13:101–108
Lance JW (1980) The control of muscle tone, reflexes, and movement: Robert Wartenberg lecture. Neurology 30:1303–1313
Latash ML, Penn RD, Corcos DM, Gottlieb GL (1989) Short-term effects of intrathecal baclofen in spasticity. Exp Neurol 103:165–172
Li S, Francisco GE (2015) New insights into the pathophysiology of post-stroke spasticity. Front Hum Neurosci 9:192. https://doi.org/10.3389/fnhum.2015.00192
Li Y, Gorassini MA, Bennett DJ (2004) Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91:767–783
Llewellyn M, Yang JF, Prochazka A (1990) Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res 83:22–28
Lundberg A, Malmgren K, Schomburg ED (1978) Role of joint afferents in motor control exemplified by effects on reflex pathways from Ib afferents. J Physiol 284:327–343. https://doi.org/10.1113/jphysiol.1978.sp012543
Magladery JW, Porter WE, Park AM, Teasdall RD (1951) Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bull Johns Hopkins Hosp 88:499–519
Mailis A, Ashby P (1990) Alterations in group Ia projections to motoneurons following spinal lesions in humans. J Neurophysiol 64:637–647
Makihara Y, Segal RL, Wolpaw JR, Thompson AK (2012) H-reflex modulation in the human medial and lateral gastrocnemii during standing and walking. Muscle Nerve 45:116–125. https://doi.org/10.1002/mus.22265
Makihara Y, Segal RL, Wolpaw JR, Thompson AK (2014) Operant conditioning of the soleus H-reflex does not induce long-term changes in the gastrocnemius H-reflexes and does not disturb normal locomotion in humans. J Neurophysiol 112:1439–1446. https://doi.org/10.1152/jn.00225.2014
Maupas E, Marque P, Roques CF, Simonetta-Moreau M (2004) Modulation of the transmission in group II heteronymous pathways by tizanidine in spastic hemiplegic patients. J Neurol Neurosurg Psychiatry 75:130–135
Maynard FM, Karunas RS, Waring WP 3rd (1990) Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil 71:566–569
Mazzaro N, Grey MJ, Sinkjaer T (2005) Contribution of afferent feedback to the soleus muscle activity during human locomotion. J Neurophysiol 93:167–177. https://doi.org/10.1152/jn.00283.2004
Mazzaro N, Grey MJ, do Nascimento OF, Sinkjaer T (2006) Afferent-mediated modulation of the soleus muscle activity during the stance phase of human walking. Exp Brain Res 173:713–723. https://doi.org/10.1007/s00221-006-0451-5
Mazzaro N, Nielsen JF, Grey MJ, Sinkjaer T (2007) Decreased contribution from afferent feedback to the soleus muscle during walking in patients with spastic stroke. J Stroke Cerebrovasc Dis 16:135–144. https://doi.org/10.1016/j.jstrokecerebrovasdis.2007.01.003
McKeon B, Burke D (1983) Muscle spindle discharge in response to contraction of single motor units. J Neurophysiol 49:291–302
Mirbagheri MM (2015) Comparison between the therapeutic effects of robotic-assisted locomotor training and an anti-spastic medication on spasticity. Conf Proc IEEE Eng Med Biol Soc 2015:4675–4678. https://doi.org/10.1109/EMBC.2015.7319437
Mirbagheri MM, Barbeau H, Ladouceur M, Kearney RE (2001) Intrinsic and reflex stiffness in normal and spastic, spinal cord injured subjects. Exp Brain Res 141:446–459
Mirbagheri MM, Chen D, Rymer WZ (2010) Quantification of the effects of an alpha-2 adrenergic agonist on reflex properties in spinal cord injury using a system identification technique. J Neuroeng Rehabil 7:29. https://doi.org/10.1186/1743-0003-7-29
Misiaszek JE (2003) The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 28:144–160. https://doi.org/10.1002/mus.10372
Morita H, Petersen N, Christensen LO, Sinkjaer T, Nielsen J (1998) Sensitivity of H-reflexes and stretch reflexes to presynaptic inhibition in humans. J Neurophysiol 80:610–620
Morita H, Shindo M, Momoi H, Yanagawa S, Ikeda S, Yanagisawa N (2006) Lack of modulation of Ib inhibition during antagonist contraction in spasticity. Neurology 67:52–56. https://doi.org/10.1212/01.wnl.0000223399.59212.f4
Mrachacz-Kersting N, Lavoie BA, Andersen JB, Sinkjaer T (2004) Characterisation of the quadriceps stretch reflex during the transition from swing to stance phase of human walking. Exp Brain Res 159:108–122
Nakajima T, Mezzarane RA, Hundza SR, Komiyama T, Zehr EP (2014) Convergence in reflex pathways from multiple cutaneous nerves innervating the foot depends upon the number of rhythmically active limbs during locomotion. PLoS One 9:e104910. https://doi.org/10.1371/journal.pone.0104910
Nakazawa K, Kawashima N, Akai M (2006) Enhanced stretch reflex excitability of the soleus muscle in persons with incomplete rather than complete chronic spinal cord injury. Arch Phys Med Rehabil 87:71–75. https://doi.org/10.1016/j.apmr.2005.08.122
Nardone A, Schieppati M (1998) Medium-latency response to muscle stretch in human lower limb: estimation of conduction velocity of group II fibres and central delay. Neurosci Lett 249:29–32
Nichols TR (2018) Distributed force feedback in the spinal cord and the regulation of limb mechanics. J Neurophysiol 119:1186–1200. https://doi.org/10.1152/jn.00216.2017
Nielsen JF, Sinkjaer T (2000) Peripheral and central effect of baclofen on ankle joint stiffness in multiple sclerosis. Muscle Nerve 23:98–105
Nielsen J, Sinkjaer T, Toft E, Kagamihara Y (1994) Segmental reflexes and ankle joint stiffness during co-contraction of antagonistic ankle muscles in man. Exp Brain Res 102:350–358
Nielsen JF, Andersen JB, Barbeau H, Sinkjaer T (1998) Input-output properties of the soleus stretch reflex in spastic stroke patients and healthy subjects during walking. NeuroRehabilitation 10:151–166. https://doi.org/10.3233/NRE-1998-10207
Nielsen JF, Anderson JB, Sinkjaer T (2000) Baclofen increases the soleus stretch reflex threshold in the early swing phase during walking in spastic multiple sclerosis patients. Mult Scler 6:105–114
Nielsen JB, Petersen NT, Crone C, Sinkjaer T (2005) Stretch reflex regulation in healthy subjects and patients with spasticity. Neuromodulation 8:49–57. https://doi.org/10.1111/j.1094-7159.2005.05220.x
Nielsen JB, Crone C, Hultborn H (2007) The spinal pathophysiology of spasticity–from a basic science point of view. Acta Physiol (Oxf) 189:171–180
Rossi A, Mazzocchio R, Scarpini C (1990) Clonus in man: a rhythmic oscillation maintained by a reflex mechanism. Electroencephalogr Clin Neurophysiol 75:56–63
Schafer SS, Dadfar F, Hartel J, Haupts S, Fischer M (1999) The period of latency before a muscle receptor generates an action potential as a response to a muscle stretch. Brain Res 843:36–47
Schieppati M (1987) The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol 28:345–376
Schieppati M, Nardone A (1997) Medium-latency stretch reflexes of foot and leg muscles analysed by cooling the lower limb in standing humans. J Physiol 503(Pt 3):691–698
Schneider C, Lavoie BA, Capaday C (2000) On the origin of the soleus H-reflex modulation pattern during human walking and its task-dependent differences. J Neurophysiol 83:2881–2890
Shefner JM, Berman SA, Sarkarati M, Young RR (1992) Recurrent inhibition is increased in patients with spinal cord injury. Neurology 42:2162–2168
Simonsen EB, Dyhre-Poulsen P (1999) Amplitude of the human soleus H reflex during walking and running. J Physiol 515(Pt 3):929–939
Sinkjaer T, Andersen JB, Larsen B (1996a) Soleus stretch reflex modulation during gait in humans. J Neurophysiol 76:1112–1120
Sinkjaer T, Andersen JB, Nielsen JF (1996b) Impaired stretch reflex and joint torque modulation during spastic gait in multiple sclerosis patients. J Neurol 243:566–574
Sinkjaer T, Andersen JB, Nielsen JF, Hansen HJ (1999) Soleus long-latency stretch reflexes during walking in healthy and spastic humans. Clin Neurophysiol 110:951–959
Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB (2000) Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523(Pt 3):817–827
Skold C, Levi R, Seiger A (1999) Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil 80:1548–1557
Stein RB, Capaday C (1988) The modulation of human reflexes during functional motor tasks. Trends Neurosci 11:328–332
Stein RB, Thompson AK (2006) Muscle reflexes in motion: how, what, and why? Exerc Sport Sci Rev 34:145–153
Stein RB, Yang JF, Belanger M, Pearson KG (1993) Modification of reflexes in normal and abnormal movements. Prog Brain Res 97:189–196
Taylor S, Ashby P, Verrier M (1984) Neurophysiological changes following traumatic spinal lesions in man. J Neurol Neurosurg Psychiatry 47:1102–1108
Thompson AK, Chen XY, Wolpaw JR (2009a) Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29:5784–5792. https://doi.org/10.1523/JNEUROSCI.4326-08.2009
Thompson AK, Estabrooks KL, Chong S, Stein RB (2009b) Spinal reflexes in ankle flexor and extensor muscles after chronic central nervous system lesions and functional electrical stimulation. Neurorehabil Neural Repair 23:133–142. https://doi.org/10.1177/1545968308321067
Thompson AK, Chen XY, Wolpaw JR (2013a) Soleus H-reflex operant conditioning changes the H-reflex recruitment curve. Muscle Nerve 47:539–544. https://doi.org/10.1002/mus.23620
Thompson AK, Pomerantz FR, Wolpaw JR (2013b) Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33:2365–2375. https://doi.org/10.1523/JNEUROSCI.3968-12.2013
Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, Craighead H (1999) Cerebral astrocyte response to micromachined silicon implants. Exp Neurol 156:33–49. https://doi.org/10.1006/exnr.1998.6983
Turns LJ, Neptune RR, Kautz SA (2007) Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch Phys Med Rehabil 88:1127–1135. https://doi.org/10.1016/j.apmr.2007.05.027
Uysal H, Larsson LE, Efendi H, Burke D, Ertekin C (2009) Medium-latency reflex response of soleus elicited by peroneal nerve stimulation. Exp Brain Res 193:275–286. https://doi.org/10.1007/s00221-008-1621-4
Uysal H, Boyraz I, Yagcioglu S, Oktay F, Kafali P, Tonuk E (2011) Ankle clonus and its relationship with the medium-latency reflex response of the soleus by peroneal nerve stimulation. J Electromyogr Kinesiol 21:438–444. https://doi.org/10.1016/j.jelekin.2010.11.005
Vincent JA, Gabriel HM, Deardorff AS, Nardelli P, Fyffe REW, Burkholder T, Cope TC (2017) Muscle proprioceptors in adult rat: mechanosensory signaling and synapse distribution in spinal cord. J Neurophysiol 118:2687–2701. https://doi.org/10.1152/jn.00497.2017
Wallace DM, Ross BH, Thomas CK (2005) Motor unit behavior during clonus. J Appl Physiol 99:2166–2172. https://doi.org/10.1152/japplphysiol.00649.2005
Wallace DM, Ross BH, Thomas CK (2012) Characteristics of lower extremity clonus after human cervical spinal cord injury. J Neurotrauma 29:915–924. https://doi.org/10.1089/neu.2010.1549
Willerslev-Olsen M, Andersen JB, Sinkjaer T, Nielsen JB (2014) Sensory feedback to ankle plantar flexors is not exaggerated during gait in spastic hemiplegic children with cerebral palsy. J Neurophysiol 111:746–754. https://doi.org/10.1152/jn.00372.2013
Yang JF, Stein RB (1990) Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol 63:1109–1117
Yang JF, Whelan PJ (1993) Neural mechanisms that contribute to cyclical modulation of the soleus H-reflex in walking in humans. Exp Brain Res 95:547–556
Yang JF, Fung J, Edamura M, Blunt R, Stein RB, Barbeau H (1991) H-reflex modulation during walking in spastic paretic subjects. Can J Neurol Sci 18:443–452
Zehr PE (2002) Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 86:455–468. https://doi.org/10.1007/s00421-002-0577-5
Zehr EP, Kido A (2001) Neural control of rhythmic, cyclical human arm movement: task dependency, nerve specificity and phase modulation of cutaneous reflexes. J Physiol 537:1033–1045
Zehr EP, Loadman PM (2012) Persistence of locomotor-related interlimb reflex networks during walking after stroke. Clin Neurophysiol 123:796–807. https://doi.org/10.1016/j.clinph.2011.07.049
Zehr EP, Stein RB (1999a) Interaction of the Jendrassik maneuver with segmental presynaptic inhibition. Exp Brain Res 124:474–480
Zehr EP, Stein RB (1999b) What functions do reflexes serve during human locomotion? Prog Neurobiol 58:185–205
Zehr EP, Komiyama T, Stein RB (1997) Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol 77:3311–3325
Zehr EP, Fujita K, Stein RB (1998a) Reflexes from the superficial peroneal nerve during walking in stroke subjects. J Neurophysiol 79:848–858
Zehr EP, Stein RB, Komiyama T (1998b) Function of sural nerve reflexes during human walking. J Physiol 507:305–314
Zuur AT (2013) Human locomotion and the motor cortex. In: Center for Sensory-Motor Interaction, Department of Health Science and Technology, Ph.D. Aalborg University, Aalborg, Denmark
Acknowledgements
This work was supported in part by the South Carolina Spinal Cord Injury Research Fund (AKT) and National Institutes of Health (NIGMS) Institutional Development Award (IDeA) U54-GM104941 (Binder-MacLeod) and NS069551 (NINDS) (AKT) and Juhl Memorial Scholarship Denmark (NM-K, TS, and JBA). We thank Dr. Jonathan R. Wolpaw for comments on this manuscript, and Ms. Stephanie Pudlik and Ms. Christina Gill for assistance in subject recruitment and data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests, financial or otherwise.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Thompson, A.K., Mrachacz-Kersting, N., Sinkjær, T. et al. Modulation of soleus stretch reflexes during walking in people with chronic incomplete spinal cord injury. Exp Brain Res 237, 2461–2479 (2019). https://doi.org/10.1007/s00221-019-05603-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05603-1