Abstract
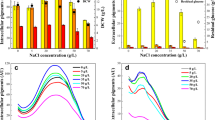
Glycerol is the principal by-product of biodiesel production which can be utilized by fungi for beneficial metabolites production. In this study, effects of glycerol on main metabolites production and gene expression of pigment and monacolin K (MK) synthesis-related genes of Monascus pilosus MS-1, a high-MK-producing but citrinin-free strain, were investigated. The results indicated that addition of glycerol could increase the dry cell weight but decrease the fermentation broth pH value. Pigments production could be enhanced significantly after adding 2 g/L glycerol and the maximum pigments yield (13.78 U/mL) of fermentation broth was obtained when the glycerol content was up to 40 g/L (p < 0.05). Effect of glycerol on MK production was different from that of Monascus pigment. MK yield of fermentation broth was decreased significantly when the glycerol addition was up to 10 g/L while that of mycelia could be improved as glycerol addition increased (p < 0.05). Results of real-time PCR revealed that when the glycerol additions were up to 40 and 300 g/L, respectively, the gene expressions of regulatory gene (ctnR) for pigment synthesis were 17.31- and 22.22-folds of ctnR in the control; the gene expressions of structural gene (PKS5) for pigment synthesis were 33.27- and 60.07-folds of PKS5 in the control; respectively. However, the gene expressions of regulatory (mkH) and structural (mkA) genes of MS-1 for MK production were decreased after adding glycerol. All this suggesting that glycerol can be used for improving production of citrinin-free Monascus pigments and MK.





Similar content being viewed by others
References
Chatzifragkou A, Papanikolaou S (2012) Effect of impurities in biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes. Appl Microbiol Biotechnol 95(1):13–27
Lu LP, Zhang BB, Xu GR (2013) Efficient conversion of high concentration of glycerol to monacolin K by solid-state fermentation of Monascus purpureus using bagasse as carrier. Bioprocess Biosyst Eng 36(3):293–299
Dobson R, Gray V, Rumbold K (2012) Microbial utilization of crude glycerol for the production of value-added products. J Ind Microbiol Biotechnol 39(2):217–226
Miyake T, Uchitomi K, Zhang MY, Kono I, Nozaki N, Sammoto H, Inagaki K (2006) Effects of the principal nutrients on lovastatin production by Monascus pilosus. Biosci Biotech Bioch 70(5):1154–1159
Tallapragada P, Dikshit R, Dessai PT (2013) Effect of glycerol as a sole carbon source on Monascus sp. for pigment production. Int Food Res J 20(6):3265–3268
Bühler RMM, Dutra AC, Vendruscolo F, Moritz DE, Ninow JL (2013) Monascus pigment production in bioreactor using a co-product of biodiesel as substrate. Food Sci Technol (Campinas) 33:9–13
Turner WB (1971) Fungal metabolites, vol 15. Academic Press, London, pp 445–476
Li L, Shao YC, Li Q, Yang S, Chen FS (2010) Identification of Mga1, a G-protein α-subunit gene involved in regulating citrinin and pigment production in Monascus ruber M7. FEMS Microbiol Lett 308(2):108–114
Shi YC, Pan TM (2011) Beneficial effects of Monascus purpureus NTU 568-fermented products: a review. Appl Microbiol Biotechnol 90(4):1207–1217
Jůzlová P, Martínková L, Křen V (1996) Secondary metabolites of the fungus Monascus: a review. J Ind Microbiol Biot 16(3):163–170
Feng YL, Shao YC, Chen FS (2012) Monascus pigments. Appl Microbiol Biotechnol 96(6):1421–1440
Kim C, Jung H, Kim YO, Shin CS (2006) Antimicrobial activities of amino acid derivatives of monascus pigments. FEMS Microbiol Lett 264(1):117–124
Patakova P (2013) Monascus secondary metabolites: production and biological activity. J Ind Microbiol Biot 40(2):169–181
Akihisa T, Tokuda H, Ukiya M, Kiyota A, Yasukawa K, Sakamoto N, Kimura Y, Suzuki T, Takayasu J, Nishino H (2005) Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem Biodivers 2(10):1305–1309
Su NW, Lin YL, Lee MH, Ho CY (2005) Ankaflavin from Monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on Hep G2 cells. J Agric Food Chem 53(6):1949–1954
Zheng Y, Xin Y, Shi X, Guo Y (2010) Cytotoxicity of Monascus pigments and their derivatives to human cancer cells. J Agric Food Chem 58(17):9523–9528
Kim JH, Kim HJ, Kim C, Jung H, Kim YO, Ju JY, Shin CS (2007) Development of lipase inhibitors from various derivatives of Monascus pigment produced by Monascus fermentation. Food Chem 101(1):357–364
Kim JH, Kim HJ, Park HW, Youn SH, Choi DY, Shin CS (2007) Development of inhibitors against lipase and α-glucosidase from derivatives of Monascus pigment. FEMS Microbiol Lett 276(1):93–98
Velmurugan P, Kim MJ, Park JS, Karthikeyan K, Lakshmanaperumalsamy P, Lee KJ, Park YJ, Oh BT (2010) Dyeing of cotton yarn with five water soluble fungal pigments obtained from five fungi. Fiber Polym 11(4):598–605
Velmurugan P, Kamala Kannan S, Balachandar V, Lakshmanaperumalsamy P, Chae JC, Oh BT (2010) Natural pigment extraction from five filamentous fungi for industrial applications and dyeing of leather. Carbohydr Polym 79(2):262–268
Calvo C, Salvador A (2002) Comparative study of the colorants Monascus and cochineal used in the preparation of gels made with various gelling agents. Food Hydrocolloid 16:523–526
Sang-aroon W, Saekow S, Amornkitbamrung V (2012) Density functional theory study on the electronic structure of Monascus dyes as photosensitizer for dye-sensitized solar cells. J Photochem Photobiol A 236:35–40
Ito S, Saitou T, Imahori H, Uehara H, Hasegawa N (2010) Fabrication of dye-sensitized solar cells using natural dye for food pigment: monascus yellow. Energy Environ Sci 3(7):905–909
Endo A (1980) Monacoiln K, a new hypocholesterolemic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme a reductase. J Antibiot XXXIII(3):334–336
Ho BY, Pan TM (2009) The Monascus metabolite monacolin K reduces tumor progression and metastasis of Lewis lung carcinoma cells. J Agric Food Chem 57(18):8258–8265
Valera HR, Gomes J, Lakshmi S, Gururaja R, Suryanarayan S, Kumar D (2005) Lovastatin production by solid state fermentation using Aspergillus flavipes. Enzyme Microb Tech 37(5):521–526
Subhasree RS, Babu PD, Vidyalakshmi R, Mohan VC (2011) Effect of carbon and nitrogen sources on stimulation of pigment produciton by Monascus purpureus on Jackfruit seeds. Intl J Microbiol Res 2(2):184–187
Said F, Brooks J, Chisti Y (2014) Optimal C: N ratio for the production of red pigments by Monascus ruber. World J Microbiol Biotechnol 30(9):2471–2479
Bühler RMM, Dutra AC, Vendruscolo F, Moritz DE, Ninow JL (2013) Monascus pigment production in bioreactor using a co-product of biodiesel as substrate. Food Sci Technol (Campinas) 33(1):9–13
Rashmi D, Padmavathi T (2013) Exploring Monascus sanguineus as a potential natural source for pigment production. Int Res J Biological Sci 2(5):59–67
Babitha S, Soccol CR, Pandey A (2007) Effect of stress on growth, pigment production and morphology of Monascus sp. in solid cultures. J Basic Microbiol 47(2):118–126
Feng YL, Shao YC, Zhou YX, Chen FS (2014) Monacolin K production by citrinin-free Monascus pilosus MS-1 and fermentation process monitoring. Eng Life Sci 14(5):538–545
Feng YL, Shao YC, Zhou YX, Chen FS (2014) Production and optimization of monacolin K by citrinin-free Monascus pilosus MS-1 in solid-state fermentation using non-glutinous rice and soybean flours as substrate. Eur Food Res Technol 239(4):629–636
Li ZQ, Guo F (2003) Morphology and taxology of Monascus spp. China Light Industry Press, Beijing
Chen MH, Johns MR (1993) Effect of pH and nitrogen source on pigment production by Monascus purpureus. Appl Microbiol Biotechnol 40(1):132–138
Orozco SFB, Kilikian BV (2008) Effect of pH on citrinin and red pigments production by Monascus purpureus CCT3802. World J Microb Biot 24(2):263–268
Dikshit R, Tallapragada P (2013) Comparative study of Monascus sanguineus and Monascus purpureus for red pigment production under stress condition. Int Food Res J 20(3):1235–1238
Carels M, Shepherd D (1977) The effect of different nitrogen sources on pigment production and sporulation of Monascus species in submerged, shaken culture. Can J Microbiol 23(10):1360–1372
Kang B, Zhang X, Wu Z, Qi H, Wang Z (2013) Effect of pH and nonionic surfactant on profile of intracellular and extracellular Monascus pigments. Process Biochem 48(5–6):759–767
Hajjaj H, Niederberger P, Duboc P (2001) Lovastatin biosynthesis by Aspergillus terreus in a chemically defined medium. Appl Environ Microb 67(6):2596–2602
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31330059, 31171649, 31271834, and 31371824).
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, Y., Shao, Y., Zhou, Y. et al. Effects of glycerol on pigments and monacolin K production by the high-monacolin K-producing but citrinin-free strain, Monascus pilosus MS-1. Eur Food Res Technol 240, 635–643 (2015). https://doi.org/10.1007/s00217-014-2365-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2365-y