Abstract
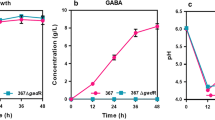
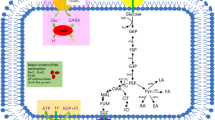
In this study, we investigated the contribution of the microbial acid stress tolerance mechanism glutamate decarboxylase (GAD) system to hop tolerance and concomitant maintenance of intracellular pH (pHin) in Lactobacillus brevis. In L. brevis, the GAD system comprises a transcriptional regulator (Gad-tr), a glutamate γ-aminobutyrate antiporter (GadC) and two glutamate decarboxylases (GadB1, GadB2). Hop iso-α-acids act as ionophores, which impair cells’ proton motive force. Hop-tolerant bacteria must therefore express effective mechanisms of pH maintenance such as the GAD system. To elucidate the specific roles of the two Gad isoenzymes, we examined the influence of iso-α-acids on the GAD system on a metabolic and transcriptional level of two L. brevis strains. Highly hop-tolerant L. brevis TMW 1.465 proved to perform better in maintenance of pHin in the presence of glutamate under hop stress when compared to the rather hop-sensitive strain L. brevis TMW 1.6. The transcriptional analysis unravelled the up- or downregulation of gad-tr, gadB 1 and gadC in hop-tolerant and hop-sensitive L. brevis, respectively. Since gadB 2 expression remained fairly unaltered, we concluded that L. brevis TMW 1.6 employs both Gad isoenzymes under acid stress, whereas L. brevis TMW 1.465 manages to survive with only one isoform (GadB2) and can consequently master additional hop stress better by inducing gadB 1 . These findings elucidate the GAD system’s role in high and low tolerance to antimicrobial hop components.







Similar content being viewed by others
References
Aiba H, Adhya S, de Crombrugghe B (1981) Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem 256:11905–11910
Albert A (1985) Selective toxicity. In: The physico-chemical basis of therapy, Chap 10. Chapman and Hall, London, pp 327–429
Arena MP, Romano A, Capozzi V, Beneduce L, Ghariani M, Grieco F, Lucas P, Spano G (2011) Expression of Lactobacillus brevis IOEB 9809 tyrosine decarboxylase and agmatine deiminase genes in wine correlates with substrate availability. Lett Appl Microbiol 53:395–402
Back W (1994) Farbatlas und Handbuch der Getränkebiologie. Nuernberg, Germany
Bartóak T, Szalai G, Lőrincz Z, Bőurcsök G, Sági F (1994) High-speed RP-HPLC/FL analysis of amino acids after automated two-step derivatization with o-phthaldialdehyde/3-mercaptopropionic acid and 9-fluorenylmethyl chloroformate. J Liq Chromatogr Relat Technol 17:4391–4403
Behr J, Gänzle MG, Vogel RF (2006) Characterization of a highly hop-resistant Lactobacillus brevis strain lacking hop transport. Appl Environ Microbiol 72:6483–6492
Behr J, Vogel RF (2009) Mechanisms of hop inhibition: hop ionophores. J Agric Food Chem 57:6074–6081
Berg JM, Tymoczko JL, Stryer L (2003) Biochemie. Spektrum Akademischer Verlag, Heidelberg
Charalampopoulos D, Pandiella S, Webb C (2003) Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int J Food Microbiol 82:133–141
Cotter PD, Gahan CG, Hill C (2001) A glutamate decarboxylase system protects Listeria monocytogenes in gastric fluid. Mol Microbiol 40:465–475
Cotter PD, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453
DeMoss RD, Bard RC, Gunsalus IC (1951) The mechanism of the heterolactic fermentation: a new route of ethanol formation. J Bacteriol 62:499–511
Duary RK, Batish VK, Grover S (2012) Relative gene expression of bile salt hydrolase and surface proteins in two putative indigenous Lactobacillus plantarum strains under in vitro gut conditions. Mol Biol Rep 39:2541–2552
García-Villalba R, Cortacero-Ramírez S, Segura-Carretero A, Martín-Lagos Contreras JA, Fernández-Gutiérrez A (2006) Analysis of hop acids and their oxidized derivatives and iso-alpha-acids in beer by capillary electrophoresis-electrospray ionization mass spectrometry. J Agric Food Chem 54:5400–5409
Haseleu G, Intelmann D, Hofmann T (2009) Structure determination and sensory evaluation of novel bitter compounds formed from β-acids of hop (Humulus lupulus L.) upon wort boiling. Food Chem 116:71–81
Higuchi T, Hayashi H, Abe K (1997) Exchange of glutamate and gamma-aminobutyrate in a Lactobacillus strain. J Bacteriol 179:3362–3364
Intelmann D, Hofmann T (2010) On the autoxidation of bitter-tasting iso-alpha-acids in beer. J Agric Food Chem 58:5059–5067
Jaskula B, Kafarski P, Aerts G, De Cooman L (2008) A kinetic study on the isomerization of hop alpha-acids. J Agric Food Chem 56:6408–6415
Kandler O (1983) Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 49:209–224
Kashket ER (1987) Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol Lett 46:233–244
Krulwich TA, Lewinson O, Padan E, Bibi E (2005) Do physiological roles foster persistence of drug/multidrug-efflux transporters? A case study. Nature reviews. Microbiology 3:566–572
Krämer J (2002) Lebensmittel-Mikrobiologie. Verlag Eugen Ulmer, Stuttgart
Lagerborg VA, Clapper WE (1952) Amino acid decarboxylases of lactic acid bacteria. J Bacteriol 63:393–397
Li H, Cao Y (2010) Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 39:1107–1116
Li H, Qiu T, Gao D, Cao Y (2010) Medium optimization for production of gamma-aminobutyric acid by Lactobacillus brevis NCL912. Amino Acids 38:1439–1445
Ma D, Lu P, Yan C, Fan C, Yin P, Wang J, Shi Y (2012) Structure and mechanism of a glutamate-GABA antiporter. Nature 483:632–636
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Rodríguez C, Rimaux T, Fornaguera MJ, Vrancken G, de Valdez GF, De Vuyst L, Mozzi F (2012) Mannitol production by heterofermentative Lactobacillus reuteri CRL 1101 and Lactobacillus fermentum CRL 573 in free and controlled pH batch fermentations. Appl Microbiol Biotechnol 93:2519–2527
Sakamoto K (2002) Beer spoilage bacteria and hop resistance in Lactobacillus brevis. University of Groningen, Netherlands
Sakamoto K, Konings WN (2003) Beer spoilage bacteria and hop resistance. Int J Food Microbiol 89:105–124
Shimwell JL (1937) On the relation between the staining properties of bacteria and their reaction towards hop antiseptic. Part I, II. J Inst Brew 43:111–118
Simpson WJ (1993) Ionophoric action of trans-isohumulone on Lactobacillus brevis. J Gen Microbiol 139:1041–1045
Simpson WJ (1993) Studies on the sensitivity of lactic acid bacteria to hop bitter acids. J Inst Brew 99:405–411
Simpson WJ, Fernandez JL (1994) Mechanism of resistance of lactic acid bacteria to trans-isohumulone. J Am Soc Brew Chem 52(1):9–11
Simpson WJ, Fernandez JL (1992) Selection of beer-spoilage lactic acid bacteria and induction of their ability to grow in beer. Lett Appl Microbiol 14:13–16
Simpson WJ, Smith ARW (1992) Factors affecting antibacterial activity of hop compounds and their derivatives. J Appl Bacteriol 72:327–334
Small PL, Waterman SR (1998) Acid stress, anaerobiosis and gadCB: lessons from Lactococcus lactis and Escherichia coli. Trends Microbiol 6:214–216
Teuber M, Schmalreck AF (1973) Membrane leakage in Bacillus subtilis 168 induced by the hop constituents lupulone, humulone, isohumulone and humulinic acid. Arch Mikrobiol 94:159–171
Toro M, Arzt E, Cerbòn J, Alegría G, Alva R, Meas Y, Estrada-O S (1987) Formation of ion-translocating oligomers by Nigericin. J Membr Biol 95:1–8
Van der Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maquin E (2002) Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82:187–216
Acknowledgments
This research project was supported by the German Ministry of Economics and Technology (via AiF) and the Wifoe (Wissenschaftsförderung der Deutschen Brauwirtschaft e.V., Berlin) project AiF 16124N. Hop iso-α-acids were kindly provided by Simon H. Steiner Hopfen GmbH, Mainburg, Germany.
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schurr, B.C., Behr, J. & Vogel, R.F. Role of the GAD system in hop tolerance of Lactobacillus brevis . Eur Food Res Technol 237, 199–207 (2013). https://doi.org/10.1007/s00217-013-1980-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-013-1980-3