Abstract
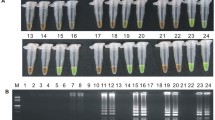
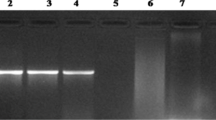
The cry1Ab gene is a foreign gene which encodes Bt insecticidal Cry1Ab protein and was transferred into genomic DNA of plants to acquire insect resistance. Here a loop-mediated isothermal amplification (LAMP) assay with high specificity and rapidity under isothermal conditions was developed for detecting cry1Ab gene in transgenic rice. Partial sequence of cry1Ab gene was used as the target template to design LAMP primers. The reaction conditions were optimized as follows: 60 min of reaction time, 1:3 of outer primer and inner primer concentration ratio, 25 μL of reaction volume and 0.6 μM of betaine. The results of detection could be evaluated by the white precipitate or the fluorescence intensity under ultraviolet irradiation, both visible to naked eyes. The sensitivity and specificity of the LAMP assay were further analyzed in comparison with that of regular PCR and real-time PCR. The results showed that the LAMP assay exhibited high specificity and the sensitivity of 3 × 102 copies number of the positive control plasmid, and of 0.5 % genetically modified (GM) contents. In comparison with real-time PCR method, LAMP showed the same results with simple instruments. The amplified reaction could be accomplished in about 1 h, with the results visible to naked eyes. Hence, the LAMP assay developed by this study can provide a rapid and simple approach for detecting cry1Ab gene from transgenic rice plants and rice ingredients in processed foods aimed at screening the growing transgenic crops in the fields and detecting genetically modified (GM) ingredients in imported and domestic foods.







Similar content being viewed by others
Abbreviations
- LAMP:
-
Loop-mediated isothermal amplification
- GMO:
-
Genetically modified organism
- GM:
-
Genetically modified
- EU:
-
European Union
- ELISA:
-
Enzyme-linked immunosorbent assay
- FIP:
-
Forward inner primer
- F1c:
-
Complementary sequence of F1
- BIP:
-
Backward inner primer
- B1c:
-
Complementary sequence of B1
- EB:
-
Ethidium bromide
- PC:
-
Positive control
- NC:
-
Negative control
References
CERA (2012) GM crop database. center for environmental risk assessment (CERA). ILSI Research Foundation, Washington DC. http://cera-gmc.org/index.php?action=gm_crop_database
James C (2011) Global status of commercialized biotech/GM crops: 2011. ISAAA brief no. 43. ISAAA, Ithaca, NY
Davison J (2010) GM plants: science, politics and EC regulations. Plant Sci 178(2):94–98
European C (2003) Commission regulation (EC) no. 1829/2003 of September 22, 2003, concerning on genetically modified food and feed. Off J Eur Commun L 268:1–23
Jennings JC, Albee LD, Kolwyck DC, Surber JB, Taylor ML, Hartnell GF, Lirette RP, Glenn KC (2003) Attempts to detect transgenic and endogenous plant DNA and transgenic protein in muscle from broilers fed YieldGard Corn Borer Corn. Poult Sci 82(3):371–380
Anklam E (1999) The validation of methods based on polymerase chain reaction for the detection of genetically modified organisms in food. Anal Chim Acta 393(1–3):177–179
Chowdhury EH, Kuribara H, Hino A, Sultana P, Mikami O, Shimada N, Guruge KS, Saito M, Nakajima Y (2003) Detection of corn intrinsic and recombinant DNA fragments and Cry1Ab protein in the gastrointestinal contents of pigs fed genetically modified corn Bt11. Am Soc Anim Sci 81(10):2546–2551
Forte VT, Di Pinto A, Martino C, Tantillo GM, Grasso G, Schena FP (2005) A general multiplex-PCR assay for the general detection of genetically modified soya and maize. Food Control 16(6):535–539
Choi S, Oh Y, Kwon J, Lee S, Han B, Ryu K (2010) Development of detection system using multiplex PCR and liquid beadarray for stacked genetically modified rice event (LS28 × Cry1Ac). J Korean Soc Appl Biol Chem 53(5):639–646
Douville M, Gagné F, Blaise C, André C (2007) Occurrence and persistence of Bacillus thuringiensis (Bt) and transgenic Bt corn cry1Ab gene from an aquatic environment. Ecotoxicol Environ Saf 66(2):195–203
Rønning SB, Vaïtilingom M, Berdal KG, Holst-Jensen A (2003) Event specific real-time quantitative PCR for genetically modified Bt11 maize (Zea mays). Eur Food Res Technol 216(4):347–354
Guertler P, Huber I, Pecoraro S, Busch U (2012) Development of an event-specific detection method for genetically modified rice Kefeng 6 by quantitative real-time PCR. Journal Verbrauch Lebensm 7(1):63–70
Raymond P, Gendron L, Khalf M, Paul S, Dibley KL, Bhat S, Xie VRD, Partis L, Moreau M-E, Dollard C, Coté M-J, Laberge S, Emslie KR (2010) Detection and identification of multiple genetically modified events using DNA insert fingerprinting. Anal Bioanal Chem 396(6):2091–2102
Liu J, Xing D, Shen X, Zhu D (2004) Detection of genetically modified organisms by electrochemiluminescence PCR method. Biosens Bioelectron 20(3):436–441
Liu J, Xing D, Shen X, Zhu D (2005) Electrochemiluminescence polymerase chain reaction detection of genetically modified organisms. Anal Chim Acta 537(1–2):119–123
Mariotti E, Minunni M, Mascini M (2002) Surface plasmon resonance biosensor for genetically modified organisms detection. Anal Chim Acta 453(2):165–172
Ahmed FE (2002) Detection of genetically modified organisms in foods. Trends Biotechnol 20(5):215–223
de Luis R, Ma Lavilla, Sénchez L, Calvo M, Pérez MAD (2010) Pepsin degradation of Cry1A(b) protein purified from genetically modified maize (Zea mays). J Agric Food Chem 58(4):2548–2553
Lutz B, Wiedemann S, Einspanier R, Mayer J, Albrecht C (2005) Degradation of Cry1Ab protein from genetically modified maize in the bovine gastrointestinal tract. J Agric Food Chem 53(5):1453–1456
Brunnert HJ, Spener F, Börchers T (2001) PCR-ELISA for the CaMV-35S promoter as a screening method for genetically modified Roundup Ready soybeans. Eur Food Res Technol 213(4):366–371
Perlak FJ, Deaton RW, Armstrong TA, Fuchs RL, Sims SR, Greenplate JT, Fischhoff DA (1990) Insect resistant cotton plants. Nature Biotechnol 8:939–943
Perlak FJ, Fuchs RL, Dean DA, McPherson SL, Fischhoff DA (1991) Modification of the coding sequence enhances plant expression of insect control protein genes. Natl Acad Sci 88:3324–3328
Sardana R, Dukiandjiev S, Giband M, Cheng X, Cowan K, Sauder C, Altosaar I (1996) Construction and rapid testing of synthetic and modified toxin gene sequences CrylA (b & c) by expression in maize endosperm culture. Plant Cell Rep 15:677–681
Prodromou C, Pearl LH (1992) Recursive PCR: a novel technique for total gene synthesis. Protein Eng 5:827–829
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgen Res 5(3):213–218
Xiang Y, Liang Z, Gao M, Shu Q, Ye G, Cheng X, Altosaar I (1999) Agrobacterium mediated transformation of insecticidal Bacillus thuringiensis cryIA(b) and cryIA(c) genes and their expression in rice. Chin J Biotechnol 15:494–500
Wang Z, Shu Q, Ye G, Cui H, Wu D, Altosaar I, Xia Y (2002) Genetic analysis of resistance of Bt rice to stripe stem borer (Chilo suppressalis). Euphytica 123(3):379–386
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucl Acids Res 28(10):e63
Fu S, Qu G, Guo S, Ma L, Zhang N, Zhang S, Gao S, Shen Z (2011) Applications of Loop-Mediated Isothermal DNA Amplification. Appl Biochem Biotechnol 163(7):845–850
Hirayama H, Kageyama S, Takahashi Y, Moriyasu S, Sawai K, Onoe S, Watanabe K, Kojiya S, Notomi T, Minamihashi A (2006) Rapid sexing of water buffalo (Bubalus bubalis) embryos using loop-mediated isothermal amplification. Theriogenology 66(5):1249–1256
Horibe D, Ochiai T, Shimada H, Tomonaga T, Nomura F, Gun M, Tanizawa T, Hayashi H (2007) Rapid detection of metastasis of gastric cancer using reverse transcription loop-mediated isothermal amplification. Int J Cancer 120(5):1063–1069
Misawa Y, Yoshida A, Saito R, Yoshida H, Okuzumi K, Ito N, Okada M, Moriya K, Koike K (2007) Application of loop-mediated isothermal amplification technique to rapid and direct detection of methicillin-resistant Staphylococcus aureus (MRSA) in blood cultures. J Infect Chemother 13(3):134–140
Qiao YM, Guo YC, Zhang XE, Zhou YF, Zhang ZP, Wei HP, Yang RF, Wang DB (2007) Loop-mediated isothermal amplification for rapid detection of Bacillus anthracis spores. Biotechnol Lett 29(12):1939–1946
Liu X, Fang J, Zhang M, Wang X, Wang W, Gong Y, Xi X, Li M (2012) Development of a loop-mediated isothermal amplification assay for detection of Cronobacter spp. (Enterobacter sakazakii). World J Microbiol Biotechnol 28(3):1013–1020
Hong M, Zha L, Fu W, Zou M, Li W, Xu D (2012) A modified visual loop-mediated isothermal amplification method for diagnosis and differentiation of main pathogens from Mycobacterium tuberculosis complex. World J Microbiol Biotechnol 28(2):523–531
Fukuta S, Mizukami Y, Ishida A, Ueda J, Hasegawa M, Hayashi I, Hashimoto M, Kanbe M (2004) Real-time loop-mediated isothermal amplification for the CaMV-35S promoter as a screening method for genetically modified organisms. Eur Food Res Technol 218(5):496–500
Lee D, La Mura M, Allnutt T, Powell W (2009) Detection of genetically modified organisms (GMOs) using isothermal amplification of target DNA sequences. BMC Biotechnol 9:7
Guan XY, Guo JC, Shen P, Yang LT, Zhang DB (2010) Visual and rapid detection of two genetically modified soybean events using loop-mediated isothermal amplification method. Food Anal Methods 3(4):313–320
Shu Q, Ye G, Cui H, Cheng X, Xiang Y, Wu D, Gao M, Xia Y, Hu C, Sardana R, Altosaar I (2000) Transgenic rice plants with a synthetic cry1Ab gene from Bacillus thuringiensis were highly resistant to eight lepidopteran rice pest species. Mol Breed 6(4):433–439
Burns M, Corbisier P, Wiseman G, Valdivia H, McDonald P, Bowler P, Ohara K, Schimmel H, Charels D, Damant A, Harris N (2006) Comparison of plasmid and genomic DNA calibrants for the quantification of genetically modified ingredients. Eur Food Res Technol 224(2):249–258
Tao ZY, Zhou HY, Xia H, Xu S, Zhu HW, Culleton RL, Han ET, Lu F, Fang Q, Gu YP (2011) Adaptation of a visualized loop-mediated isothermal amplification technique for field detection of Plasmodium vivax infection. Parasites Vectors 4:115
Babekova R, Funk T, Pecoraro S, Engel KH, Busch U (2009) Development of an event-specific Real-time PCR detection method for the transgenic Bt rice line KMD1. Eur Food Res Technol 228:707–716
Luan F, Tao R, Xu Y, Wu J, Guan X (2012) High-throughput detection of genetically modified rice ingredients in foods using multiplex polymerase chain reaction coupled with high-performance liquid chromatography method. Eur Food Res Technol 234(4):649–654
Zhang L, Yang JB, Song FS, Chen S, Guo X, Li L, Wang XF (2008) Detecting of Bt transgenic rice by real-time quantitative PCR technique. J Biol 25(6):63–71
Reiting R, Grohmann L, Mäde D (2010) A testing cascade for the detection of genetically modified rice by real-time PCR in food and its application for detection of an unauthorized rice line similar to KeFeng6. J Verbrauch Lebensm 5(2):185–188
Su CQ, Xie JJ, Wang XF, Peng YF (2011) Integrated structure and event-specific real-time detection of transgenic cry1Ac/SCK rice Kefeng 6. Eur Food Res Technol 232(2):351–359
Acknowledgments
This work was supported by the Natural Science Foundation Programs of Zhejiang Province (Y3090617, Y304463), the Innovation Team of Zhejiang Province (No. 2010R50028) and the Key Science and Technological Achievement Transformation Project of Zhejiang Province (No. 2011E61018), China. The authors thank Dr. Mingzhe Zhang (Zhejiang Entry-exit Inspection and Quarantine Institute of Science and Technology, China) for providing GM samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Q., Fang, J., Liu, X. et al. Loop-mediated isothermal amplification (LAMP) method for rapid detection of cry1Ab gene in transgenic rice (Oryza sativa L.). Eur Food Res Technol 236, 589–598 (2013). https://doi.org/10.1007/s00217-013-1911-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-013-1911-3