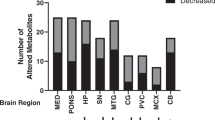
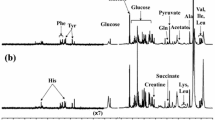
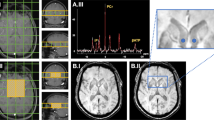
Abstract
In vivo proton magnetic resonance spectroscopy (1H-MRS) and matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) are two semi-quantitative analytical methods commonly used in neurochemical research. In this study, the two methods were used complementarily, in parallel, to investigate neurochemical perturbations in the medial prefrontal cortex (mPFC) of 9-month-old DJ-1 knockout mice, a well-established transgenic model for Parkinson’s diseases. Convergingly, the results obtained with the two methods demonstrated that, compared with the wild-type (WT) mice, the DJ-1 knockout mice had significantly increased glutathione (GSH) level and GSH/glutamate (Glu) ratio in the mPFC, which likely presented an astrocytic compensatory mechanism in response to elevated regional oxidative stress induced by the loss of DJ-1 function. The results from this study also highlighted (1) the need to be cautious when interpreting the in vivo 1H-MRS results obtained from aged transgenic animals, in which the concentration of internal reference, being whether water or total creatine, could no longer be assumed to be the same as that in the age-matched WT animals, and (2) the necessity and importance of complementary analyses with more than one method under such circumstances.
Graphical abstract






Similar content being viewed by others
Data availability
The data set will be available upon request.
Abbreviations
- 1H-MRS:
-
Proton magnetic resonance spectroscopy
- MRI:
-
Magnetic resonance imaging
- MALDI-MSI:
-
Matrix-assisted laser desorption/ionization mass spectrometry imaging
- 6-OHDA:
-
6-Hydroxydopamine
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- WT:
-
Wild-type
- CRLB:
-
Cramér–Rao lower bound
- tCr:
-
Total creatine
- Cr:
-
Creatine
- PCr:
-
Phosphocreatine
- NAA:
-
N-Acetyl aspartate
- Tau:
-
Taurine
- Cys:
-
Cysteine
- DA:
-
Dopaminergic
- Gln:
-
Glutamine
- Glu:
-
Glutamate
- Gly:
-
Glycine
- GSH:
-
Glutathione
- MFB:
-
Medial forebrain bundles
- mPFC:
-
Medial prefrontal cortex
- SNc:
-
Substantia nigra pars compacta
- FrA:
-
Frontal association cortex
- ROI:
-
Region of interest
- SNR:
-
Signal-to-noise ratio
- FWHM:
-
Full width at half maximum
References
Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–48.
George JL, Mok S, Moses D, Wilkins S, Bush AI, Cherny RA, et al. Targeting the progression of Parkinson’s disease. Curr Neuropharmacol. 2009;7(1):9–36.
Lee MR, Denic A, Hinton DJ, Mishra PK, Choi D-S, Pirko I, et al. Preclinical 1H-MRS neurochemical profiling in neurological and psychiatric disorders. Bioanalysis. 2012;4(14):1787–804.
Jenner P, Olanow CW. Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology. 1996;47(6):S161–70.
González-Rodríguez P, Zampese E, Stout KA, Guzman JN, Ilijic E, Yang B, et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature. 2021;599(7886):650–6.
Benshachar D, Riederer P, Youdim MBH. Iron-melanin interaction and lipid peroxidation: implications for Parkinson’s disease. J Neurochem. 1991;57(5):1609–14.
Mailly F, Marin P, Israel M, Glowinski J, Premont J. Increase in external glutamate and NMDA receptor activation contribute to H2O2-induced neuronal apoptosis. J Neurochem. 1999;73(3):1181–8.
Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC, et al. Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochem Res. 2011;36(8):1452–63.
Kumar J, Liddle EB, Fernandes CC, Palaniyappan L, Hall EL, Robson SE, et al. Glutathione and glutamate in schizophrenia: a 7T MRS study. Mol Psychiatry. 2018;25(4):873–82.
Duarte JMN, Lei H, Mlynárik V, Gruetter R. The neurochemical profile quantified by in vivo 1H NMR spectroscopy. Neuroimage. 2012;61(2):342–62.
Alger JR. Quantitative proton magnetic resonance spectroscopy and spectroscopic imaging of the brain: a didactic review. Top Magn Reson Imaging. 2010;21(2):115–28.
Pearce RKB, Owen A, Daniel S, Jenner P, Marsden CD. Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J Neural Transm. 1997;104(6–7):661–77.
Smeyne RJ, Gröger A, Kolb R, Schäfer R, Klose U. Dopamine reduction in the substantia nigra of Parkinson’s disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS ONE. 2014;9(1):e84081.
Toczylowska B, Zieminska E, Michałowska M, Chalimoniuk M, Fiszer U. Changes in the metabolic profiles of the serum and putamen in Parkinson’s disease patients – in vitro and in vivo NMR spectroscopy studies. Brain Res. 2020;1748:147118.
Chassain C, Bielicki G, Keller C, Renou JP, Durif F. Metabolic changes detected in vivo by 1H MRS in the MPTP-intoxicated mouse. NMR Biomed. 2010;23(6):547–53.
Heo H, Ahn J-B, Lee HH, Kwon E, Yun J-W, Kim H, et al. Neurometabolic profiles of the substantia nigra and striatum of MPTP-intoxicated common marmosets: an in vivo proton MRS study at 9.4 T. NMR Biomed. 2017;30(2):e3686.
Coune PG, Craveiro M, Gaugler MN, Mlynarik V, Schneider BL, Aebischer P, et al. An in vivo ultrahigh field 14.1 T 1H-MRS study on 6-OHDA and alpha-synuclein-based rat models of Parkinson’s disease: GABA as an early disease marker. NMR Biomed. 2013;26(1):43–50.
Kickler N, Lacombe E, Chassain C, Durif F, Krainik A, Farion R, et al. Assessment of metabolic changes in the striatum of a rat model of parkinsonism: an in vivo 1H MRS study. NMR Biomed. 2009;22(2):207–12.
Hou Z, Zhang Z, Meng H, Lin X, Sun B, Lei H, et al. Parkinson’s disease: in vivo metabolic changes in the frontal and parietal cortices in 6-OHDA treated rats during different periods. Int J Neurosci. 2014;124(2):125–32.
Bagga P, Crescenzi R, Krishnamoorthy G, Verma G, Nanga RP, Reddy D, et al. Mapping the alterations in glutamate with GluCEST MRI in a mouse model of dopamine deficiency. J Neurochem. 2016;139(3):432–9.
Ren X, Hinchie A, Swomley A, Powell DK, Butterfield DA. Profiles of brain oxidative damage, ventricular alterations, and neurochemical metabolites in the striatum of PINK1 knockout rats as functions of age and gender: relevance to Parkinson disease. Free Radical Biol Med. 2019;143:146–52.
Helms G. The principles of quantification applied to in vivo proton MR spectroscopy. Eur J Radiol. 2008;67(2):218–29.
Norris JL, Caprioli RM. Analysis of tissue specimens by matrix-assisted laser desorption/ionization imaging mass spectrometry in biological and clinical research. Chem Rev. 2013;113(4):2309–42.
Schubert KO, Weiland F, Baune BT, Hoffmann P. The use of MALDI-MSI in the investigation of psychiatric and neurodegenerative disorders: a review. Proteomics. 2016;16(11–12):1747–58.
Rzagalinski I, Volmer DA. Quantification of low molecular weight compounds by MALDI imaging mass spectrometry – a tutorial review. Biochim Biophys Acta (BBA) - Proteins Proteom. 2017;1865(7):726–39.
Linscheid MW. Molecules and elements for quantitative bioanalysis: the allure of using electrospray, MALDI, and ICP mass spectrometry side-by-side. Mass Spectrom Rev. 2019;38(2):169–86.
Wu H, Liu X, Gao Z-Y, Lin M, Zhao X, Sun Y, et al. Icaritin provides neuroprotection in Parkinson’s disease by attenuating neuroinflammation, oxidative stress, and energy deficiency. Antioxidants. 2021;10(4):529.
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–9.
Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104(37):14807–12.
Meiser J, Delcambre S, Wegner A, Jager C, Ghelfi J, d’Herouel AF, et al. Loss of DJ-1 impairs antioxidant response by altered glutamine and serine metabolism. Neurobiol Dis. 2016;89:112–25.
Lopert P, Patel M. Brain mitochondria from DJ-1 knockout mice show increased respiration-dependent hydrogen peroxide consumption. Redox Biol. 2014;2:667–72.
de Hemptinne C, Chen WT, Racine CA, Seritan AL, Miller AM, Yaroshinsky MS, et al. Prefrontal physiomarkers of anxiety and depression in Parkinson’s disease. Front Neurosci. 2021;15:748165.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013.
Li M, Xu H, Chen G, Sun S, Wang Q, Liu B, et al. Impaired D2 receptor-dependent dopaminergic transmission in prefrontal cortex of awake mouse model of Parkinson’s disease. Brain. 2019;142(10):3099–115.
Chen L, Cagniard B, Mathews T, Jones S, Koh HC, Ding Y, et al. Age-dependent motor deficits and dopaminergic dysfunction in DJ-1 null mice. J Biol Chem. 2005;280(22):21418–26.
Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press; 2003.
Moffett JR, Namboodiri MAA. Differential distribution of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat forebrain. J Neurocytol. 1995;24(6):409–33.
Moffett JR, Namboodiri MAA, Cangro CB, Neale JH. Immunohistochemical localization of N-acetylaspartate in rat brain. NeuroReport. 1991;2(3):131–4.
Fonnum F. Regulation of the synthesis of the transmitter glutamate pool. Prog Biophys Mol Biol. 1993;60(1):47–57.
Zhang H, Zou Y, Lei H. Regional metabolic differences in rat prefrontal cortex measured with in vivo 1H-MRS correlate with regional histochemical differences. NMR Biomed. 2019;32(1):e4024.
O’Neill J, Schuff N, Marks WJ, Feiwell R, Aminoff MJ, Weiner MW. Quantitative 1H magnetic resonance spectroscopy and MRI of Parkinson’s disease. Mov Disord. 2002;17(5):917–27.
Harish G, Mahadevan A, Srinivas Bharath MM, Shankar SK. Alteration in glutathione content and associated enzyme activities in the synaptic terminals but not in the non-synaptic mitochondria from the frontal cortex of Parkinson’s disease brains. Neurochem Res. 2012;38(1):186–200.
Garrido M, Tereshchenko Y, Zhevtsova Z, Taschenberger G, Bahr M, Kugler S. Glutathione depletion and overproduction both initiate degeneration of nigral dopaminergic neurons. Acta Neuropathol. 2011;121(4):475–85.
Kumar P, Kaundal RK, More S, Sharma SS. Beneficial effects of pioglitazone on cognitive impairment in MPTP model of Parkinson’s disease. Behav Brain Res. 2009;197(2):398–403.
Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700.
Raman AV, Chou VP, Atienza-Duyanen J, Di Monte DA, Bellinger FP, Manning-Boğ AB. Evidence of oxidative stress in young and aged DJ-1-deficient mice. FEBS Lett. 2013;587(10):1562–70.
Takahashi-Niki K, Inafune A, Michitani N, Hatakeyama Y, Suzuki K, Sasaki M, et al. DJ-1-dependent protective activity of DJ-1-binding compound no. 23 against neuronal cell death in MPTP-treated mouse model of Parkinson’s disease. J Pharmacol Sci. 2015;127(3):305–10.
Lev N, Barhum Y, Ben-Zur T, Melamed E, Steiner I, Offen D. Knocking out DJ-1 attenuates astrocytes neuroprotection against 6-hydroxydopamine toxicity. J Mol Neurosci. 2013;50(3):542–50.
González-Polo RA, Niso-Santano M, Morán JM, Ortiz-Ortiz MA, Bravo-San Pedro JM, Soler G, et al. Silencing DJ-1 reveals its contribution in paraquat-induced autophagy. J Neurochem. 2009;109(3):889–98.
De Miranda BR, Rocha EM, Bai Q, El Ayadi A, Hinkle D, Burton EA, et al. Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson’s disease. Neurobiol Dis. 2018;115:101–14.
Björkblom B, Adilbayeva A, Maple-Grødem J, Piston D, Ökvist M, Xu XM, et al. Parkinson disease protein DJ-1 binds metals and protects against metal-induced cytotoxicity. J Biol Chem. 2013;288(31):22809–20.
Aoyama K, Nakaki T. Impaired glutathione synthesis in neurodegeneration. Int J Mol Sci. 2013;14(10):21021–44.
Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain - metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267(16):4912–6.
Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98(3):641–53.
Ramírez-García G, Palafox-Sánchez V, Limón ID. Nitrosative and cognitive effects of chronic l-DOPA administration in rats with intra-nigral 6-OHDA lesion. Neuroscience. 2015;290:492–508.
Stanojlovic M, Pallais Yllescas JP, Vijayakumar A, Kotz C. Early sociability and social memory impairment in the A53T mouse model of Parkinson’s disease are ameliorated by chemogenetic modulation of orexin neuron activity. Mol Neurobiol. 2019;56(12):8435–50.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 21790392 and 21790390).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences (protocol number: APM20028A).
Source of biological material
DJ-1 knockout mice (C57BL/6 J background) were kindly provided by the Experimental Animal Center of Peking University and bred and maintained at the Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences.
Statement of animal welfare
All animal experiments were performed according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, W., Liu, H., Liu, S. et al. Altered prefrontal neurochemistry in the DJ-1 knockout mouse model of Parkinson’s disease: complementary semi-quantitative analyses with in vivo magnetic resonance spectroscopy and MALDI-MSI. Anal Bioanal Chem 414, 7977–7987 (2022). https://doi.org/10.1007/s00216-022-04341-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04341-8