Abstract
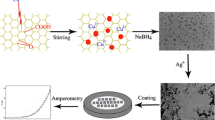
Hybrids of silver nanoparticle-decorated reduced graphene oxide (Ag-RGO) have been prepared with the use of poly(ionic liquid) (PIL) as a versatile capping agent to develop volatile organic compound (VOC) sensors. The hybrid materials of Ag-RGO/PIL were assembled into three-dimensional-laminated nanostructures, where spherical Ag nanoparticles with diameters between 50 and 300 nm were homogeneously distributed on the graphene sheets and interspaced between them. Ag-RGO/PIL sensors were fabricated by spray layer-by-layer technique and used to detect a set of polar (methanol, ethanol, methyl acetate, acetone and water) and non-polar (chloroform, dichlorobenzene, toluene and styrene) organic vapours. Much higher sensitivity and discriminability were obtained for polar vapours although non-polar ones could also be detected. In comparison with either simple reduced graphene oxide or carbon nanotubes (CNT) functionalised by PIL, the hybrid Ag-RGO/PIL-based sensors showed superior performances in terms of sensitivity, selectivity, stability and high reliability. For example, a signal-to-noise ratio up to 168 was obtained for 1 ppm of methanol and signals drift between two experiments spaced out in the time of 3 months was less than 3 %. It is expected that by extrapolation, a limit of detection at the parts per billion level can be reached. These results are promising to design e-noses based on high stability chemoresistive sensors for emerging applications such as anticipated diagnostic of food degradation or diseases by the analysis of VOC, some of them being in this case considered as biomarkers.






Similar content being viewed by others
References
Radushkevich LV, Lukyanovich MV (1952) About the structure of carbon formed by thermal decomposition of carbon monoxide on iron substrate. J Phys Chem (Moscow) 26:88–95
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Baughman RH, Zakhidov AA, De Heer WA (2002) Carbon nanotubes—the route toward applications. Science 297:787–892
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H (2000) Nanotube molecular wires as chemical sensors. Science 287:622–625
Feller JF, Castro M, Kumar B (2011) Polymer CNT conductive nanocomposite for sensing. In: Mc Nally T (ed) Polymer carbon nanotube composites: preparation, properties and applications, Chap. 25. Whoodhead Publishing Ltd, Cambridge, UK, p 848. ISBN 1845697618
Feller JF, Castro M, Kumar B (2011) Conductive biopolymer nanocomposites for sensors. In: Mittal V (ed) Nanocomposites with biodegradable polymers: synthesis, properties and future perspectives, Chap. 15. Oxford University Press, Oxford, UK, p. 440, ISBN 978-0-19-958192-4
Chatterjee S, Castro M, Feller JF (2013) An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J Mater Chem B 1:4563–4575
Chen G, Paronyan TM, Pigos EM, Harutyunyan AR (2012) Enhanced gas sensing in pristine carbon nanotubes under continuous ultraviolet light illumination. Sci Rep 2(343):1–7
Castro M, Lu J, Bruzaud S, Kumar B, Feller JF (2009) Carbon nanotubes/poly(ε-caprolactone) composite vapour sensors. Carbon 47:1930–1942
Kumar B, Castro M, Feller JF (2012) Controlled conductive junction gap for chitosan—carbon nanotubes quantum resistive vapour sensors. J Mater Chem 22:10656–10664
Kostarelos K (2008) The long and short of carbon nanotube toxicity. Nature Biotech 26:774–776
Donaldson K, Poland CA (2009) Nanotoxicology: new insights into nanotubes. Nature Nanotech 4:708–710
Nerl HC, Cheng C, Goode AE, Bergin SD, Lich B, Gass M, Porter AE (2011) Imaging methods for determining uptake and toxicity of carbon nanotubes in vitro and in vivo. Nanomedicine 6:849–865
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA (2005) Two-dimensional gas of massless Dirac fermions in graphene. Nature 438:197–200
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Rirsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Gilje S, Han S, Wang MS, Wang KL, Kaner RB (2007) A chemical route to graphene for device applications. Nano Lett 7:3394–3398
Kim TY, Lee HW, Stoller M, Dreyer DR, Bielawski CW, Ruoff RS, Suh KS (2011) High-performance supercapacitors based on poly(ionic liquid)-modified graphene electrodes. ACS Nano 5:436–442
Choi HJ, Jung SM, Seo JM, Chang DW, Dai L, Baek JB (2012) Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 1:534–551
Dua V, Surwade SP, Ammu S, Agnihotra SR, Jain S, Roberts KE, Park S, Ruoff RS, Manohar SK (2010) All-organic vapour sensor using inkjet-printed reduced graphene oxide. Angew Chem 49:1–5
Robinson JT, Perkins FK, Snow ES, Wei Z, Sheehan PE (2008) Reduced graphene oxide molecular sensors. Nano Lett 8:3137–3140
Han TH, Huang YK, Tan ATL, Dravid VP, Huang J (2011) Steam etched porous graphene oxide network for chemical sensing. J Am Chem Soc 33:15264–15267
Dan Y, Lu Y, Kybert NJ, Luo Z, Johnson ATC (2009) Intrinsic response of graphene vapour sensors. Nano Lett 9:1472–1475
Tung TT, Castro M, Kim TY, Suh KS, Feller JF (2012) Graphene quantum resistive sensing skin for the detection of alteration biomarkers. J Mater Chem 22:21754–21766
Xu C, Wang X, Zhu JW (2008) Graphene-metal particle nanocomposites. Phys Chem C 112:19841–19845
Vedala H, Sorescu DC, Kotchey GP, Star A (2011) Chemical sensitivity of graphene edges decorated with metal nanoparticles. Nano Lett 11:2342–2347
Scheuermann GM, Rumi L, Steurer P, Bannwarth W, Mülhaupt R (2009) Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki–Miyaura coupling reaction. J Am Chem Soc 131:8262–8270
Goncalves G, Marques PAAP, Granadeiro CM, Nogueira HIS, Singh MK, Gracio J (2009) Surface modification of graphene nanosheets with gold nanoparticles: the role of oxygen moieties at graphene surface on gold nucleation and growth. Chem Mater 21:4796–4802
Si Y, Samulski ET (2008) Exfoliated graphene separated by platinum nanoparticles. Chem Mater 20:6792–6797
Li J, Liu C (2010) Ag/graphene heterostructures: synthesis, characterization and optical properties. Eur J Inorg Chem 8:1244–1248
Tien HW, Huang YL, Yang SY, Wang JY, Ma CCM (2011) The production of graphene nanosheets decorated with silver nanoparticles for use in transparent, conductive films. Carbon 49:1550–1560
Yang YK, He CE, He WJ, Yu LJ, Peng RG, Xie XL, Wang XB, Mai YW (2011) Reduction of silver nanoparticles onto graphene oxide nanosheets with N,N-dimethylformamide and SERS activities of GO/Ag composites. J Nanopart Res 13:5571–5581
Shen J, Shi M, Li N, Yan B, Ma H, Hu Y, Ye M (2010) Facile synthesis and application of Ag-chemically converted graphene nanocomposite. Nano Res 3:339–349
Pasricha R, Gupta S, Srivastava AK (2009) A facile and novel synthesis of Ag–graphene-based nanocomposites. Small 5:2253–2259
Bao Q, Zhang D, Qi P (2011) Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J Colloid Interface Sci 360:463–470
Xu Z, Gao H, Guoxin H (2011) Solution-based synthesis and characterization of a silver nanoparticle–graphene hybrid film. Carbon 49:4731–4738
Lee J, Novoselov KS, Shin HS (2011) Interaction between metal and graphene: dependence on the layer number of graphene. ACS Nano 5:608–612
Zhang Y, Liu S, Wang L, Qin X, Tian J, Lu W, Chang G, Sun X (2012) One-pot green synthesis of Ag nanoparticles-graphene nanocomposites and their applications in SERS, H2O2, and glucose sensing. RSC Adv 2:538–545
Zhang Z, Xu F, Yang W, Guo M, Wang X, Zhang B, Tang J (2011) A facile one-pot method to high-quality Ag–graphene composite nanosheets for efficient surface-enhanced Raman scattering. Chem Commun 47:6440–6442
Tung TT, Kim TY, Shim JP, Yang WS, Kim H, Suh KS (2011) Poly(ionic liquid)-stabilized graphene sheets and their hybrid with poly(3,4-ethylenedioxythiophene). Org Electro 12:2215–2224
Tung TT, Feller JF, Kim TY, Kim H, Yang WS, Suh KS (2012) Electromagnetic properties of Fe3O4-functionalized graphene and its composites with a conducting polymer. J Polym Sci A: Polym Chem 50:927–935
Marcilla R, Blazquez JA, Rodríguez J, Pomposo JA, Mecerreyes D (2004) Tuning the solubility of polymerized ionic liquids by simple anion-exchange reactions. J Polym Sci A: Polym Chem 42:208–212
Marcilla R, Blazquez JA, Fernandez R, Grande H, Pomposo JA, Mecerreyes D (2005) Synthesis of novel polycations using the chemistry of ionic liquids. Macromol Chem Phys 206:299–304
Kim TY, Lee TH, Kim JE, Kasi RM, Sung CSP, Suh KS (2008) Organic solvent dispersion of poly(3,4-ethylenedioxythiophene) with the use of polymeric ionic liquid. J Polym Sci A: Polym Chem 46:6872–6879
Bouvree A, Feller JF, Castro M, Grohens Y, Rinaudo M (2009) Conductive polymer nano-biocomposites (CPC): chitosan-carbon nanoparticle a good candidate to design polar vapour sensors. Sensors Actuators B Chem 138:138–147
Lu J, Park BJ, Kumar B, Castro M, Choi HJ, Feller JF (2010) Polyaniline nanoparticle–carbon nanotube hybrid network vapour sensors with switchable chemo-electrical polarity. Nanotechnology 21:1–10
Kim TY, Lee HW, Kim JE, Suh KS (2010) Synthesis of phase transferable graphene sheets using ionic liquid polymers. ACS Nano 4:1612–1618
Zhou Y, Bao Q, Tang LAL, Zhong Y, Loh KP (2009) Hydrothermal dehydration for the “green” reduction of exfoliated graphene oxide to graphene and demonstration of tunable optical limiting properties. Chem Mater 21:2950–2956
Lin Z, Yao Y, Li Z, Liu Y, Li Z, Wong CP (2010) Solvent-assisted thermal reduction of graphite oxide. J Phys Chem C 114:14819–14825
Lu J, Feller JF, Kumar B, Castro M, Kim YS, Park YT, Grunlan JC (2011) Chemo-sensitivity of latex-based films containing segregated networks of carbon nanotubes. Sens Actuators B: Chem 155:28–36
Feller JF, Lu J, Zhang K, Kumar B, Castro M, Gatt N, Choi HJ (2011) Novel architecture of carbon nanotube decorated poly(methyl methacrylate) microbead vapour sensors assembled by spray layer by layer. J Mater Chem 21:4142–4149
Castro M, Kumar B, Feller JF, Haddi Z, Amari A, Bouchikhi B (2011) Novel e-nose for the discrimination of volatile organic biomarkers with an array of carbon nanotubes (CNT) conductive polymer nanocomposites (CPC) sensors. Sens Actuators B: Chem 159:213–219
Im J, Sengupta SK, Baruch MF, Granz CD, Ammu S, Manohar SK, Whitten JE (2011) A hybrid chemiresistive sensor system for the detection of organic vapours. Sens Actuators B: Chem 156:715–722
Gao T, Woodka MD, Brunschwig BS, Lewis NS (2006) Chemiresistors for array-based vapour sensing using composites of carbon black with low volatility organic molecules. Chem Mater 18:5193–5202
Li J, Lu Y, Ye Q, Cinke M, Han J, Meyyappan M (2003) Carbon nanotube sensors for gas and organic vapour detection. Nano Lett 3:929–933
Hakim M, Broza YY, Barash O, Peled N, Phillips M, Amann A, Haick H (2012) Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev 112:5949–5966
http://ddbonline.ddbst.de/AntoineCalculation/AntoineCalculationCGI.exe
Service du répertoire toxicologique: CSST. Available from http://www.reptox.csst.qc.ca/
Acknowledgments
This work was supported by a grant from the University of South Brittany (UBS, France). We are grateful to Isabelle Pillin, Anthony Magueresse and Hervé Béllegou for their contributions to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Chemosensors and Chemoreception with guest editors Jong-Heun Lee and Hyung-Gi Byun.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2516 kb)
Rights and permissions
About this article
Cite this article
Tung, T.T., Castro, M., Kim, T.Y. et al. High stability silver nanoparticles–graphene/poly(ionic liquid)-based chemoresistive sensors for volatile organic compounds’ detection. Anal Bioanal Chem 406, 3995–4004 (2014). https://doi.org/10.1007/s00216-013-7557-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7557-y