Abstract
The authors describe a method for room temperature preparation of reduced graphene oxide (rGO) decorated with Ag-Cu nanoparticles (NPs). The nanocomposite (Ag-CuNP/rGO) was characterized by scanning electron microscopy, energy dispersive X-ray spectroscopy, X-ray photoelectron spectroscopy and transmission electron microscopy. A glassy carbon electrode (GCE) modified with this nanocomposite dispersed is shown to be a viable electrode for determination of Sudan I (best at a working voltage of −112 mV vs. Ag/AgCl), with remarkably increased electrochemical response to Sudan I compared to that of a plain GCE. The calibration plot is linear in the 1.0 nM to 10 µM concentration range, with a 0.4 nM detection limit (at a signal-to-noise ratio of 3). The method was successfully applied to the determination of Sudan I in ketchup and chili powder.

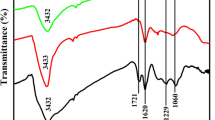
The nanocomposite of reduced graphene oxide (rGO) decorated with Ag-Cu nanoparticles (Ag-CuNP/rGO) was prepared using NaBH4 as a reductant at room temperature. A glassy carbon electrode (GCE) was coated with a Ag-CuNP/rGO nanocomposite to form a modified electrode that can detect trace concentrations of Sudan I.







Similar content being viewed by others
References
Mao Y, Fan Q, Li J, Yu L, Qu L (2014) A novel and green CTAB-functionalized graphene nanosheets electrochemical sensor for Sudan I determination. Sensors Actuators B Chem 203:759
Yu W, Liu Z, Li Q, Zhang H, Yu Y (2015) Determination of Sudan I–IV in candy using ionic liquid/anionic surfactant aqueous two-phase extraction coupled with high-performance liquid chromatography. Food Chem 173:815
Xu X, Tian X, Cai L, Xu Z, Lei H, Wang H, Sun Y (2014) Molecularly imprinted polymer based surface plasmon resonance sensors for detection of Sudan dyes. Anal Methods 6:3751
Stiborova M, Martinek V, Rydlova H, Hodek P, Frei E (2002) Sudan I is a potential carcinogen for humans: evidence for its metabolic activation and Detoxication by human recombinant cytochrome P450 1 A1 and liver Microsomes. Cancer Res 62:5678
Li JH, Feng HB, Li J, Feng YL, Zhang YQ, Jiang JB, Qian D (2015) Fabrication of gold nanoparticles-decorated reduced graphene oxide as a high performance electrochemical sensing platform for the detection of toxicant Sudan I. Electrochim Acta 167:226
Commission Decision 2003/460/EC of 20 June 2003 on emergency measures regarding hot chilli and hot chilli products (2003) Off J Eur Communities L154:114
Calbiani F, Careri M, Elviri L, Mangia A, Pistara L (2004) I. Zagnoni, development and in-house validation of a liquid chromatography–electrospray–tandem mass spectrometry method for the simultaneous determination of Sudan I, Sudan II, Sudan III and Sudan IV in hot chilli products. J Chromatogr A 1042:123
Hu XG, Fan YA, Zhang Y, Dai GM, Cai QL, Cao YJ, Guo CJ (2012) Molecularly imprinted polymer coated solid-phase microextraction fiber prepared by surface reversible addition–fragmentation chain transfer polymerization for monitoring of Sudan dyes in chilli tomato sauce and chilli pepper samples. Anal Chim Acta 731:40
Alothman ZA, Unsal YE, Habila M, Shabaka A, Tuzen M, Soylak M (2012) Membrane filtration of Sudan orange G on a cellulose acetate membrane filter for separation–preconcentration and spectrophotometric determination in water, chili powder, chili sauce and tomato sauce samples. Food Chem Toxicol 50:2709
Piao C, Chen L (2012) Separation of Sudan dyes from chilli powder by magnetic molecularly imprinted polymer. J Chromatogr A 1268:185
Taverna D, Donna LD, Mazzotti F, Policicchio B, Sindona G (2013) High-throughput determination of Sudan azo-dyes within powdered chili pepper by paper spray mass spectrometry. J Mass Spectrom 48:544
Xu XY, Tian XG, Cai LG, Xu ZL, Lei HT, Wang H, Sun YM (2014) Molecularly imprinted polymer based surface plasmon resonance sensors for detection of Sudan dyes. Anal Methods 6:3751
Ling Y, Li JX, Qu F, Li NB, Luo HQ (2014) Rapid fluorescence assay for Sudan dyes using polyethyleneimine-coated copper nanoclusters. Microchim Acta 181:1069
Elyasi M, Khalilzadeh MA, Karimi-Maleh H (2013) High sensitive voltammetric sensor based on Pt/CNTs nanocomposite modified ionic liquid carbon paste electrode for determination of Sudan I in food samples. Food Chem 141:4311
Wu YH (2010) Electrocatalysis and sensitive determination of Sudan I at the single-walled carbon nanotubes and iron(III)-porphyrin modified glassy carbon electrodes. Food Chem 121:580
Prabakaran E, Pandian K (2015) Amperometric detection of Sudan I in red chili powder samples using Ag nanoparticles decorated graphene oxide modified glassy carbon electrode. Food Chem 166:198
Geim AK, Novoselov KS (2007) The rise of grapheme. Nat Mater 6:183
Sahoo NG, Pan YZ, Li L, Chan SH (2012) Graphene-based materials for energy conversion. Adv Mater 24:4203
Kong BS, Geng J, Jung HT (2009) Layer-by-layer assembly of graphene and gold nanoparticles by vacuum filtration and spontaneous reduction of gold ions. Chem Commun 16:2174
Yang L, Wang G, Liu Y (2013) An acetylcholinesterase biosensor based on platinum nanoparticles–carboxylic graphene–nafion-modified electrode for detection of pesticides. Anal Biochem 437:144–149
Gong J, Miao X, Zhou Z, Zhang L (2011) An enzymeless organophosphate pesticide sensor using Au nanoparticle-decorated graphene hybrid nanosheet as solid-phase extraction. Talanta 85:1344
Lu XQ, Qi HT, Zhang XF, Xue ZH, Jin J, Zhou XB, Liu XH (2011) Highly dispersive Ag nanoparticles on functionalized graphene for an excellent electrochemical sensor of nitroaromatic compounds. Chem Commun 47:12494
Luo J, Jiang SS, Zhang HY, Jiang JQ, Liu XY (2012) A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal Chim Acta 709:47
Vilian ATE, Hwang SK, Kwak ChH OSY, Kim CY, Lee G, Lee Jin B, Huh YS, Han YK (2016) Pt-Au bimetallic nanoparticles decorated on reduced graphene oxide as an excellent electrocatalysts for methanol oxidation. Synth Met 219:52
Takehiro N, Liu P, Bergbreiter A, Nørskovb JK, Behm RJ (2014) Hydrogenadsorption on bimetallic Pd-Au(111) surface alloys: minimum adsorptionensemble, ligand and ensemble effects, and ensemble confinement. PhysChem Chem Phys 16:23930
Ding LX, Li GR, Wang ZL, Liu ZQ, Liu H, Tong YX (2012) Porous Ni@Pt Core-Shell nanotube Array Electrocatalyst with high activity and stability for methanol oxidation. Chem-Eur J 18:8386
Yin AX, Min XQ, Zhu W, Liu WC, Zhang YW, Yan CH (2012) Pt-Cu and Pt-Pd-Cu concave nanocubes with high-index facets and superiorelectrocatalytic activity. Chem Eur J 18:777–782
Yano H, Kataoka M, Yamashita H, Uchida H, Watanabe M (2007) Oxygenreduction activity of carbon-supported Pt-M (M = V, Ni, Cr, Co, and Fe) alloysprepared by nanocapsule method. Langmuir 23:6438
Mao H, Li RS, Jiang K, Huang T, Yu AS (2013) Facile preparation of Cu@Pt/rGO hybrids and their electrocatalyticactivities for methanol oxidation. Electrochim Acta 107:419
Sreedhar NY, Kumar MS, Krishnaveni K (2015) Sensitive determination of chlorpyrifos using Ag/Cu alloynanoparticles and graphene composite paste electrode. Sensors Actuators B 210:475
Li FH, Guo YQ, Liu Y, Qiu HX, Sun XY, Wang W, Liu Y, Gao JP (2013) Fabrication of Pt–Cu/RGO hybrids and their electrochemical performance for the oxidation of methanol and formic acid in acid media. Carbon 64:11
Mao H, Li RS, Jiang K, Huang T, Yu AS (2011) Facile preparation of Cu@Pt/rGO hybrids and their electrocatalytic activities for methanol oxidation. Electrochim Acta 107(2013):419–424
Osch THJ, Perelaer J, Laat AWM, Schubert US (2008) Inkjet printing of narrow conductive tracks on untreated polymeric substrates. Adv Mater 20:343
Li GP, Luo YJ (2008) Preparation and characterization of dendrimer-templated Ag–Cu bimetallic nanoclusters. Inorg Chem 47:360
Hummers JW, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Kang P, Bobyr E, Dustman J, Hodgson KO, Hedman B, Solomon EI, Stack TDP (2010) Bis(μ-oxo) Dicopper(III) species of the simplest Peralkylated diamine: enhanced reactivity toward exogenous substrates. Inorg Chem 49:11030
Ma LB, Shen XP, Ji ZY, Zhu GX, Zhou H (2014) Ag nanoparticles decorated MnO2/reduced graphene oxide as advanced electrode materials for supercapacitors. Chem Eng J 252:95
Jia ZF, Chen TD, Wang J, Ni JJ, Li HY, Shao X (2015) Synthesis, characterization and tribological properties of Cu/reduced graphene oxide composites. Tribol Int 88:17
Li C, Liu W, Gu Y, Hao S, Yan X, Zhang Z, Yang M (2014) Simultaneous determination of catechol and hydroquinone based on poly (sulfosalicylic acid)/functionalized graphene modified electrode. J Appl Electrochem 44:1059
Prabakaran E, Pandian K (2015) Amperometric detection of Sudan I in red chili powder samples using Ag nanoparticles decorated graphene oxide modified glassy carbon electrode. Food Chem 166:198
Duan DH, Liu HH, You X, Wei HK, Liu SB (2015) Anodic behavior of carbon supported Cu@Ag core–shell nanocatalysts in direct borohydride fuel cells. J Power Sources 293:292
Gan T, Li K, Wu K (2008) Multi-wall carbon nanotube-based electrochemical sensor for sensitive determination of Sudan I. Sensors Actuators B Chem 132:134
Yang D, Zhu L, Jiang X (2010) Electrochemical reaction mechanism and determination of Sudan I at a multi wall carbon nanotubes modified glassy carbon electrode. J Electroanal Chem 640:17
Chen S, Du D, Huang J, Zhang A, Tu H, Zhang A (2011) Rational design and application of molecularly imprinted sol–gel polymer for the electrochemically selective and sensitive determination of Sudan I. Talanta 84:451
Zhang L, Zhang X, Li X, Peng Y, Shen H, Zhang Y (2013) Determination of Sudan I using electrochemically reduced graphene oxide. Anal Lett 46:923
Wu M, Tang W, Gu J, Wang Q, He P, Fang Y (2013) Electrochemical detection of Sudan I using a multi-walled carbon nanotube/chitosan composite modified glassy carbon electrode. Am J Anal Chem 4:1
Acknowledgments
We thank the National Natural Science Foundation of China (Grant No. 20775002, 21405003) for financial support. The work was supported by Program for Innovative Research Team in Anhui Normal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 2999 kb)
Rights and permissions
About this article
Cite this article
Yao, Y., Liu, Y. & Yang, Z. Highly sensitive electrochemical sensor for the food toxicant Sudan I based on a glassy carbon electrode modified with reduced graphene oxide decorated with Ag-Cu nanoparticles. Microchim Acta 183, 3275–3283 (2016). https://doi.org/10.1007/s00604-016-1977-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1977-2