Abstract
Concentrations of Cd, Cu, Cr, Pb, Ni and Zn were monitored in the Svitava River (the Czech Republic) during April and September 2005. Total concentrations and total dissolved concentrations were obtained through regular water sampling, and the diffusive gradients in thin films technique (DGT) were used to gain information on the kinetically labile metal concentrations. Each measured concentration was compared with the corresponding average (bio)available concentration calculated from the mass of metal accumulated by the moss species Fontinalis antipyretica. The concentrations of Cd, Pb, Cr and Zn measured using DGT corresponded well with those obtained after the deployment of Fontinalis antipyretica moss bags in the Svitava River, but the concentrations of Cu and Ni did not. The calculated (bio)available Cu concentration correlated well with the total dissolved concentration of Cu, whereas no correlation was found to exist between the concentrations of Ni.

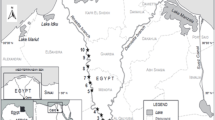
Scheme of the Svitava River monitoring station, including the DGT sampling units and Fontinalis antipyretica moss bags


Similar content being viewed by others
References
Ndungu K, Hurst, MP, Bruland KW (2005) Environ Sci Technol 39:3166–3175
Slaveykova VI, Parthasarathy N, Buffle J, Wilkinson KJ (2004) Sci Total Environ 328:55–68
Parthasarathy N, Buffle J, Gassama N, Cuenod F (1999) Chem Anal 44:455–470
Ammann AA (2002) Anal Bioanal Chem 372:448–452
Sarzanini C, Bruzzoniti MC (2001) Trends Anal Chem 20:304–310
Downard AJ, Panther J, Kim YC, Powell KJ (2003) Anal Chim Acta 499:17–28
Apte SC, Batley GE, Bowle KC, Brown PL, Creighton N, Hales LT, Hyne RV, Juli M, Markich SJ, Pablo F, Rogers NJ, Stauber JL, Wilde K (2005) Environ Chem 2:320–330
Twiss MR, Moffett JW (2002) Environ Sci Technol 36:1061–1068
Kozelka PB, Bruland KW (1998) Mar Chem 60:267–282
Davison W, Zhang H (1994) Nature 367:546–548
Zhang H, Davison W (1995) Anal Chem 67:3391–3400
Denney S, Sherwood J, Leyden J (1999) Sci Tot Environ 239:71–80
Alfaro-De la Torre MC, Beaulieu PY, Tessier A (2000) Anal Chim Acta 418:53–68
Li W, Zhao H, Teasdale PR, John R, Zhang S (2002) Anal Chim Acta 464:331–339
Li W, Teasdale PR, Zhang S, John R, Zhao H (2003) Anal Chem 75:2578–2584
Dočekalová H, Diviš P (2005) Talanta 65:1174–1178
Allen HE, Hall RH, Brisbin TD (1980) Environ Sci Technol 35:441–443
Tusseau-Vuillemin MH, Gilbin R, Bakkaus E, Garric J (2004) Environ Toxicol Chem 23:2154–2161
Zhang H, Zhao FJ, Sun B, Davison W, McGrath SP (2001) Environ Sci Technol 35:2602–2607
Meyer JS (2002) Mar Environ Res 53:417–423
Webb JA, Keough MJ (2002) Mar Pollut Bull 44:222–229
Mouvet C (1984) Environ Technol Lett 5:541–548
Mersch J, Johansson L (1993) Environ Technol 14:1027–1036
Mersch J, Reichard M (1998) Arch Environ Contam Toxicol 34:336–342
Rasmussen G, Andersen S (1999) Water Air Soil Pollut 109:41–52
Figueira R, Ribeiro T (2005) Environ Pollut 136:293–301
Goncalves EP, Boaventura RAR (1998) Wat Res 32:1305–1313
United States Environmental Protection Agency (1998) National recommended water quality criteria. Federal Register 63(234):67548–67558
Hamilton-Taylor J, Smith EJ, Davison W, Zhang H (1999) Limnol Oceanogr 44:172–1780
Eggleton J, Thomas KV (2004) Environ Int 30:973–980
Borovec Z (2000) Chem Listy 94:939–945
Bruns I, Friese K, Markert B, Krauss GJ (1997) Sci Tot Environ 204:161–176
Cenci RM (2000) J Limnol 60(Suppl 1):53–61
Samecka-Cymrman A, Kolon K, Kempers AJ (2005) Sci Tot Environ 341:97–107
Acknowledgement
The work was supported by the Ministry of Education, Youth and Sports of the Czech Republic (Projects No. MSM 0021630502 and G4/814/2005 of FRVS). J.P. Matousek is gratefully acknowledged for helpful comments and for help with manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement Table 1
Physicochemical conditions of the Svitava River at the monitoring station site during the period of the study (XLS 14kb)
Rights and permissions
About this article
Cite this article
Diviš, P., Dočekalová, H., Brulík, L. et al. Use of the diffusive gradients in thin films technique to evaluate (bio)available trace metal concentrations in river water. Anal Bioanal Chem 387, 2239–2244 (2007). https://doi.org/10.1007/s00216-006-0996-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0996-y