Abstract
The Rosetta Branch is one of Egypt’s most important Nile River branches, providing freshwater to multiple cities. However, its water quality has been deteriorating, with various wastes containing high loads of heavy metals being discharged into its body of water. Seasonally, water and sediment samples and two native aquatic plants (Ceratophyllum demersum and Eichhornia crassipes) were collected and analyzed from the Rosetta Branch to assess the level of metal contamination (Fe, Mn, Zn, Cu, Pb, Ni, Cd, Cr, and Co) using different metal indices. The levels of some metals in the branch water overstepped those suitable for drinking water and aquatic life. In increasing order, the means of the heavy metal concentrations in branch water (µg/L) were Cd (1.8–4.9) < Co (7.18–28.1) ≈ Ni (9.0–25.1) < Cr (8.56–27.4) < Cu (14–75) < Pb (9.3–67.9) < Zn (22–133) < Mn (68–220) < Fe (396–1640). All the metal indices measured in the sediment confirmed the Ni and Cd contamination, where Ni and Cd in the sediment surpass the sediment quality guidelines in 80% and 53% of samples, respectively, reflecting frequent adverse effects on aquatic organisms. According to the bioconcentration factor, C. demersum and E. crassipes have higher accumulation capacities mainly for Cd than those for other metals considered as major pollutants in the water and sediment of Rosetta Branch, reflecting the role of hydrophytes in the biological treatment of polluted water in aquatic environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rivers are important natural resources for irrigation, drinking, and domestic uses, and they help improve the economy and sustainability of nearby towns (Loucks & van Beek, 2017). The Nile River is Egypt’s lifeline; hence, its water quality is a vital issue for Egyptians. However, Egypt’s surface water quality is deteriorating because of increased anthropogenic activity, such as agricultural runoff and urban and industrial wastes (Al-Afify et al., 2018). The Nile River divides into two main branches (Rosetta and Damietta) 25 km north of Cairo and flows into the Mediterranean Sea (Ali et al., 2014). The Rosetta Branch water is of great vital importance as it serves as a water source for municipal, industrial, agricultural, navigation, and aquaculture purposes (Authman et al., 2009; Eissa et al., 2022).
In many African countries, significant population growth has been accompanied by steep increases in urbanization and industrial and agricultural land use over the last few decades (Idodo-Umeh & Oronsaye, 2006). This has resulted in the significant rise in contaminant discharge into receiving water bodies, which negatively affects the aquatic ecology. Heavy metals make up most of these contaminants, which eventually end up in bottom sediments (Abou El-Anwar et al., 2021). Pollution discharges, particularly heavy metals and nutrient salts, have severe influences on river health and the appropriateness of Nile River water for industrial, household, and agricultural purposes (Abdel-Satar et al., 2022; Al-Afify & Abdel-Satar, 2020; Othman et al., 2021).
Heavy metals are an intriguing category of elements in terms of aquatic contamination because of their severe effects on the aquatic system equilibrium, long-term persistence, and ability to accumulate in water and sediments, in addition to their bioaccumulation in living organisms (Masresha et al., 2021). Among these metals, Cd and Pb are potentially toxic and have no known role in biological systems, whereas others such as Cu and Zn are essential. Still, when the intake of essential metals by aquatic organisms is extremely high, hazardous effects can occur (Yacoub et al., 2021). High inputs of metals to aquatic systems can cause serious water pollution problems, resulting in potential risks to human health through the food chain (Othman et al., 2021).
Metal contamination in aquatic environments is escalating at alarming levels, and it has now become a major global problem. Metal pollution is typically monitored by assessing the metal levels in the water, sediments, and biotic components such as aquatic plants and fish (Ali et al., 2019). Using solely water analyses to identify metals as inputs in aquatic environments is not regarded as proper (Turkmen et al., 2006). Metal levels in sediments, in addition to the measurement of aqueous metals, play a significant role in water quality assessments (Hadi et al., 2019). Sediments are considered the most important indicators of heavy metals because they tend to absorb dissolved metals and act as potential nonpoint metal sources by releasing them into the water column (Algül & Beyhan, 2020).
Aquatic macrophytes are good biological monitors of heavy metals. They play significant roles in water quality control, oxygen production, nutrient cycling, and sediment stabilization, and they serve as aquatic life habitat and shelter (Tshithukhe et al., 2021). Metals can accumulate in high levels in aquatic macrophytes from the water or/and sediments, proving the utility of plants as aquatic biomonitors. Furthermore, monitoring the buildup of persistent contaminants in aquatic macrophytes can offer time-integrated data on harmful chemicals in aquatic systems (Harguinteguy et al., 2014). The levels of heavy metals in aquatic macrophytes depend on the plant species, the plant sections, the metal levels in the growth environment, and the macrophytes’ selective capacity to absorb different substances (Tshithukhe et al., 2021).
Pollution indices are valuable tools for measuring the level of heavy metal contamination in water, sediment, and aquatic plants. The indices help determine if metal accumulation is due to natural or anthropogenic mechanisms (Kowalska et al., 2018). As a result, determining the levels of heavy metals is critical for analyzing the Nile River environment. The present work was undertaken to (1) monitor the distribution of some essential and toxic metals in the water, sediment, and two aquatic macrophytes (Ceratophyllum demersum and Eichhornia crassipes) in the Rosetta Branch of the Nile River and (2) to perform ecological risk assessments for heavy metals in the Rosetta Branch using the pollution indices of different metals.
2 Materials and Methods
2.1 Study Area
The Nile River is Egypt’s principal source of freshwater, and its flow rate is dependent on the amount of water stored in Nasser Lake to meet the country’s annual water needs (Othman et al., 2021). The high dam at Aswan and a series of seven barrages between Aswan and the Mediterranean Sea totally control the Nile River (Al-Afify & Abdel-Satar, 2020). The Nile River is divided into two branches 25 km north of Cairo. The western branch (approximately 242 km long) is known as the Rosetta Branch, whereas the eastern branch is known as the Damietta Branch (about 239 km long). The study region covered around 123 km of the Rosetta Branch, from El-Kanater El-Khyria upstream to Kafr El-Zayat City downstream.
The Rosetta Branch is approximately 225 km long and 150 m wide, with an average depth of 2–2.3 m. Numerous sources of pollution may impact and impair the Rosetta Branch’s water quality. El-Rahawy drain (domestic) is the main source of pollution in the branch and has the potential to degrade its water quality (Othman et al., 2021). It dumps around 1.5 million cubic meters of sewage from the Giza Governorate and agricultural wastes into the water branch every day (APRP, 2002). Furthermore, the branch in Kafr El-Zayat, one of Egypt’s most important industrial regions, which comprises industrial effluents from sulfur and superphosphate compound manufacturers and soap and pesticide factories (Abd El-Hady, 2014). Also, sewage from various cities and neighboring villages is dispersed along the two banks of the Rosetta Branch, and other tiny agricultural drains dump their wastes straight into the water (Othman et al., 2021). The release of untreated sewage water into aquatic habitats has become a serious concern, resulting in declining water quality and seriously threatening aquatic life (Bastami et al., 2015).
2.2 Sample Collection, Processing, and Analysis
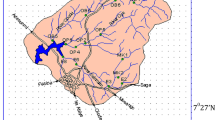
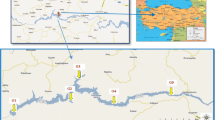
Seasonally, all samples were taken from ten locations in the Rosetta Branch from the mid-stream channel (40 samples for each media). As indicated in Table 1, the selected locations are both directly and indirectly subjected to municipal wastewater, agricultural drainage, and industrial disposals. Consequently, heavy metals, suspended matter, and organic pollutants damage the Nile system (Othman et al., 2021). Table 2 shows the basic features of the Rosetta Branch water, whereas Fig. 1 shows the sampling sites.
Map showing the sampling sites in the Rosetta Branch of the Nile River (after Othman et al., 2021)
A polyvinyl Van Dorn plastic bottle was used to collect ten subsurface (approximately 30 cm) water samples from the Rosetta Branch’s mid-stream channel. The samples were maintained in clean stoppered plastic bottles and preserved using nitric acid (65%) to a pH of less than 2. Lastly, the samples were digested using nitric acid (65%) according to APHA (2005).
Sediment samples were taken from the ten sampling sites in the Rosetta Branch using Eckman sampling equipment from the top 20-cm layer of the bottom. Stone fragments were removed by running the air-dried samples through a 2-mm sieve. The sieved sediment was powdered, where 0.5 g of finely ground samples was digested using Kouadia and Trefry’s technique (1987).
Also, according to the plant type, representative macrophytes were collected from the ten sampling sites (submerged or floating). A 0.5 m × 0.5 m quadrant was used to capture floating macrophytes (E. crassipes). Meanwhile, submerged plants (C. demersum) were harvested using a volume-controlled grab. The macrophytes were thoroughly rinsed with distilled water three times before drying at 60 °C. The dried macrophyte parts were ground, and 0.5 g was digested using 65% nitric acid, 98% sulfuric acid, and 35% hydrogen peroxide, following Saison et al. (2004).
The samples (water, sediment, and plants) were analyzed for Mn, Fe, Cu, Zn, Cd, Pb, Ni, Cr, and Co concentrations using inductively coupled plasma mass spectrometry (iCAP TQ ICP-MS; Thermo Scientific, Germany). Triplicate readings were used to control the precision of the metal analyses, where the average values were determined with less than 5% relative standard deviations.
2.3 Water Assessment
The metal content suitability of the Rosetta Branch water for drinking purposes was determined using the heavy metal pollution index (HPI) proposed by Prasad & Bose (2001) and the contamination index (Cd) proposed by Backman et al. (1997). Also, the Rosetta Branch water was assessed for drinking, irrigation, and aquatic life suitability using the pollution index (PI) proposed by Caerio et al. (2005). The metal guidelines used for the several index measurements were the Egyptian drinking water quality standards (EWQS, 2007) and that of the World Health Organization (WHO, 2017) for drinking water, that of the United State Environmental Protection Agency (USEPA) for aquatic life suitability, and those of WHO/Food and Agriculture Organization (FAO) (2007) for irrigation use. The indices used are described in detail in the supplementary materials (Text S1).
2.4 Sediment Assessment
Several indices were used to evaluate the metal contamination risk in Rosetta Branch sediment. These include the enrichment factor (EF) index (Yahaya et al., 2012); geoaccumulation index (I-geo) (Müller, 1969); potential ecological risk index (ER) (Hakanson, 1980, 1988); modified contamination degree (mCd) (Abrahim & Parker, 2008); pollution load index (PLI) (Tomlinson et al., 1980); toxic risk index (TRI) (Gao et al., 2018); and the toxic unit (Pedersen et al., 1998). The metal content guideline used for the several measurement indices was the Freshwater Sediment Screening Benchmarks (EPA, 2006; MacDonald et al., 2000). More details on these indices can be found in the supplementary file (Text S2).
2.5 Macrophyte Assessment
The bioconcentration factor (BCF) was used to assess the plants’ ability to absorb and accumulate heavy metals from aqueous media. The detailed calculation of the factor is in the supplementary file (Text S3).
2.6 Statistical Analysis
Pearson correlation coefficients (p < 0.01 and 0.05) were used to estimate the correlations between the metal concentrations in the water, sediment, and plant samples. Furthermore, data from the water and sediment samples were examined for significant spatial and temporal distributions using the analysis of variance test.
3 Results and Discussion
3.1 Heavy Metals in Surface Water
In recent decades, the metal contamination of river water has been viewed as a severe ecological and public health hazard (Lipy et al., 2021). Table 3 shows the range, mean, and standard deviation of the metal concentrations in the Rosetta Branch water during different seasons. In increasing order, the means of the heavy metal concentrations (µg/L) were Cd (1.8–4.9) < Co (7.18–28.1) ≈ Ni (9.0–25.1) < Cr (8.56–27.4) < Cu (14–75) < Pb (9.3–67.9) < Zn (22–133) < Mn (68–220) < Fe (396–1640). The metal concentrations in the branch water varied significantly by site (p < 0.05), with the highest levels of all examined metals observed at sites 3 and 6. This indicates the fluctuation of metals in sewage effluents, industrial wastes, and agricultural drainage water discharged into the branch. The branch water metal contents were compared with the WHO (2017) and EWQS (2007) guidelines for drinking water, chronic levels for aquatic life criteria (USEPA, 1993), and the WHO/FAO (2007) standard for irrigation use. The obtained results show that the levels of Fe, Pb, and Cd in most sites and throughout different seasons were higher than the EWQS (2007) and WHO (2017) threshold limits established for drinking water suitability. The Cr, Cd, and Pb levels in all locations and seasons surpassed USEPA’s chronic thresholds for aquatic life, whereas the Fe and Zn concentrations in anthropogenically impacted locations exceeded the standards. Ni is the only metal with concentrations that are still within acceptable limits for aquatic life. As confirmed by Sani et al. (2022), the increase in the metal contents in water may originate from anthropogenic activities, such as urban and agricultural runoff (fertilizers and pesticides).
Finally, except for Mn in station 6 during the spring season, all metals were within the WHO/FAO (2007) irrigation standards. In the branch water, significant correlations between all measured metal pairs (r = 0.59–0.95; n = 40, P < 0.01) revealed common metal sources and associations.
Table 3 shows the calculated HPI values for the branch locations based on the WHO (2017) and EWQS (2007) criteria. Except for sites 1 and 2, the HPI at all branch sites surveyed at different seasons exceeded the critical HPI of 100 suggested by Prasad & Bose (2001) for drinking water, indicating that the branch water is seriously contaminated with metals, according to the EWQS (2007) and WHO (2017). Except for upstream unpolluted areas (sites 1 and 2), the entire examined branch length had moderate (1 ≤ Cd ≤ 3) or high (Cd ≥ 3) metallic levels of pollution. The branch water is highly polluted by heavy metals at the anthropogenically impacted sites (3 and 6), as shown in Fig. 2. The main explanations for the regional variations in the metal levels are the amount of agricultural drainage water, sewage effluents, and industrial wastes released into the branch water through the El-Rahawy and Sobal drains, as documented by Abdel-Satar et al. (2017a) and Al-Afify & Abdel-Satar (2020) for Nile water. Extreme care must be applied at the anthropogenic input points, where the metals can bioaccumulate in aquatic organisms, to keep the heavy metal influx under control.
The PI is based on the evaluation of individual metals in the branch water. For various uses, the detected metals showed varying degrees of pollution in the branch water. According to WHO/FAO (2007), the PI value for irrigation usage showed no degree of pollution at various places for all seasons. According to the assessment of the measured metal concentrations for drinking purposes (EWQS, 2007), the branch water suffers from various contamination grades, with the PI for Zn and Cu being significantly less than 1, indicating no adverse effect. Cd had only a slight impact on the branch water at site 3 (the discharge point of the El-Rahawy drain). Finally, except for sites 1 and 2, the branch water is contaminated to varying degrees by Fe, Mn, and Pb in the following ascending order: Mn > Fe > Pb (Fig. 3).
The PI data for the aquatic life assessment show that Fe had only a minor impact on the branch water at site 6 (Sobal Drain discharge point), whereas Cr had a slight pollution impact on all sites except 1 and 2. Pb pollution was moderate to high in the branch water, but Cd pollution was low to moderate (Fig. 3).
Table 4 compares heavy metal concentrations in the Nile River’s water at Rosetta Branch to those in other global rivers, where the Rosetta Branch water has ordinary heavy metal levels, with the exception of areas in front of pollution sources. The pollution of the River Nile system by agricultural, domestic, and industrial pollutants has generally grown in recent decades due to population growth (Abdelmageed et al., 2022).
3.2 Heavy Metals in Surface Sediment
The metal levels in sediment from several sites on the Nile River’s Rosetta Branch are shown in Table 5. The heavy metal concentrations in the Rosetta Branch sediment are substantially greater than those in the water, as indicated in Tables 3 and 5, indicating metal enrichment in the Nile sediment.
In descending order, the average amounts of heavy metals in the sediments were Fe > Mn > Zn > Pb ≈ Ni > Cr ≈ Cu > Co > Cd. When compared with those in the upstream sites, the metal levels increased in the branch sediment at sites 3, 6, and 10 at different seasons and exceeded the freshwater sediment EPA benchmarks. These indicate that the sediments receive a heavy load of domestic, agricultural, and industrial waste from various sources, including the discharge points of the El-Rahawy and Sobal drains. The Pearson correlation analysis revealed the association between the heavy metal pairs Mn/Zn, Mn/Co, Mn/Cr, Mn/Cd, Zn/Ni, Zn/Co, Zn/Cd, Cu/Pb, Ni/Co, Ni/Cr, Ni/Cd, Co/Cr, Co/Cd, Pb/Cr, and Cr/Cd (r = 0.30–0.87, n = 40, p < 0.05), suggesting similar sources of metal inputs (human or natural) in the Nile sediment. No significant variations in metal levels across the sampling seasons were noted, except for Cu. Furthermore, except for Cu and Pb, the metals in the branch sediment had significant spatial distributions.
The assessment of the EF index values for the Rosetta Branch established that Ni and Cd have generally higher enrichment than those of the other metals (Fig. 4). The EF values for Cu, Pb, Ni, and Cd indicate moderate enrichment at about 3%, 5%, 18%, and 33% of the samples, respectively. The EF range of 0.5–1.5 indicated that a metal was entirely derived from natural processes or crustal minerals, whereas EF values greater than 1.5 indicated that metal sources were largely anthropogenic (Abdel-Satar et al., 2017b). An analysis of the spatial distribution of EF revealed high contamination of sediments in the downstream stretch of the Rosetta Branch, where significant spatial differences in the EF values were recorded, reflecting the effect of downstream point pollution sources.
The analysis of the average of the I-geo values confirmed the Ni and Cd pollution intensity in the samples, where the Rosetta Branch was practically unpolluted to moderately polluted in all seasons concerning Ni and Fe for most sites, especially the downstream stretch. Concerning Cd, the investigated sites were moderately polluted except the upstream sites 1 and 2, which were unpolluted. The results of the I-geo values showed that the sediment of the branch was practically unpolluted by Mn, Zn, Cu, Co, Cr, and Pb (Fig. 5).
Furthermore, as demonstrated in Fig. 6, the metal pollution in the Rosetta Branch sediment determined using the mCd and ER indices is characterized by a different spatial distribution. The presence of point and area pollution sources resulted in higher index values in the samples (sites 3, 6, and 7), and the metal contamination in the upstream sites 1 and 2 along the branch was the lowest. The mCd and ER values in all seasons for sites 3, 6, and 7 were classified as moderate-grade risk (mCd > 1.5; ER > 150). The average contribution of Cd to ER was about 80% in the branch sediment, whereas the Ni contribution was 8%, followed by a 5% Pb contribution.
Another index used to assess the contamination levels in the Rosetta Branch sediments is the PLI. The resulting PLI values are presented in Table 5 and range from 0.78 to 1.50. At all sites except sites 1, 2, and 5, the PLI index values in all seasons were greater than 1, indicating contaminated sites because of the appreciable input of metals from anthropogenic sources. Except for Cd, the contamination factor (Cf) values for all the analyzed metals revealed moderate levels of contamination (1 ≤ Cf < 3) in various sites and seasons (Table 5), but the Cf values for Cd showed slightly high levels of contamination (Cf > 3) for sites 3, 6, and 7.
The sediment quality guidelines (SQGs) give a simple and comparable technique for quantifying the level of metal pollution and its potential damage to aquatic ecosystems (Zhao et al., 2021). SQGs established for freshwater ecosystems were utilized to estimate the contamination level and potential risk of metals in sediments (MacDonald et al., 2000) using the probable effects level (PEL) and threshold effects level (TEL) (Table 5). Most sediment samples showed high levels of Zn, Cu, Pb, and Cr exceeding the TEL in 55%, 53%, 98%, and 58% of the samples, respectively, reflecting occasional adverse effects on aquatic biota inhabiting the Nile. Meanwhile, Ni and Cd in the branch sediment surpassed the PEL in 80% and 53% of the samples, respectively, indicating frequent adverse effects on aquatic organisms. Because of the significant influences of domestic wastes, industrial emissions, agricultural applications of pesticides and fertilizers, and atmospheric deposition, many sources continuously degrade the sediments (Ali et al., 2022). The overall degree of ER for the metals in the branch sediments (ƩTU and TRI) revealed that the samples posed low to moderate overall ecological risks, with no seasonal differences and high regional variations (Figs. 7 and 8). The ƩTU value for the metals was greater than 4 in sites 3 and 6 (point sources of pollution) and downstream of these sites (4 and 7), reflecting a significant toxic effect on aquatic biota (Gao et al., 2018). Similar observations were noted for the TRI values, where the anthropogenically influenced areas (sites 3, 4, 6, and 7) recorded values greater than 10, indicating a moderate level of pollution. Cd contributed about 26% and 44% to the toxic risk indices (ƩTU and TRI), respectively, whereas Ni contributed approximately 34% and 21%, and Pb contributed 14% and 11%, respectively.
3.3 Heavy Metals in Macrophytes
Bulk mineral salts can be absorbed by aquatic plants from sediments via the root system, from the water column via the leaves, or both (Abdel-Satar & Geneid, 2009). Figure 9 shows the metal levels in two macrophytes from the Rosetta Branch. Although there are limitations when comparing concentrations in different species owing to their differences in exposure and absorption ability, the average distribution of the most analyzed elements in the two plants showed similar trends: Fe > Mn > Zn > Cu > Co > Pb > Ni > Cr > Cd, where Fe, Mn, Ni, Cr, and Co were dominant in C. demersum. Significant seasonal variations were recorded for Mn, Cd, and Co in E. crassipes and for Fe, Zn, Cd, and Co in C. demersum. Also, significant spatial distributions were observed for all studied metals in the two plants, except for Cd in E. crassipes and Pb in C. demersum, where the upstream sites 1 and 2 showed the lowest accumulation levels of most metals in the two macrophytes. At the same anthropogenically impacted sites, high levels of heavy metal accumulation in E. crassipes and C. demersum were observed, with the highest metal levels seen in the surface water and sediment, where the macrophytes are considered the biological filters that significantly impact metal mobility in aquatic ecosystems. Consequently, they may be able to accumulate metals at levels greater than their contents in a given environment (Harguinteguy et al., 2014).
The levels of the examined metals were much higher in E. crassipes and C. demersum than those in the branch water. The BCF is the ratio of heavy metal levels in the plant to the same metal content in water. It is used to reflect the heavy metal capacity of plants compared with that of the surrounding water (Al-Afify & Abdel-Satar, 2020). The higher the EF, the better the plant’s ability to absorb metals (Sun & Zheng, 2018). The metal BCF values were ranked in the following order for the two studied plants (Fig. 10): Cd > Mn > Zn > Fe > Co > Cu ≈ Ni > Pb > Cr, where Cd bioaccumulation was several times higher than those of other metals. Cd, which was considered the major pollutant in the water and sediment of the Rosetta Branch, was able to accumulate in the tissues of E. crassipes and C. demersum in self-purification processes. Therefore, these species could be considered as heavy metal bioindicators of Nile pollution.
4 Conclusion
On the basis of the current findings and analysis, we can derive the following conclusions:
-
Heavy metals accumulated at varying degrees in all components (water, sediments, and plants) at sites 3, 4, 6, and 7 near the discharge points of the El-Rahawy and Sabal drains.
-
The levels of some metals in the branch water overstepped the aquatic life suitability guidelines in front of and downstream the pollution source. Also, the HPI, Cd, and PI metal indices confirmed that the levels of some metals, especially Cd and Pb, in the branch water exceeded the drinking water criteria.
-
The metal levels were the highest in the branch sediments at sites 3, 6, and 10 at different seasons and exceeded the freshwater sediment EPA benchmarks. Ni and Cd in the branch sediments surpassed the PEL of SQG in 80% and 53% of samples, respectively, reflecting frequent adverse effects on aquatic organisms.
-
The calculated EF and I-geo indices for the metals in the Rosetta Branch established that Ni and Cd have generally higher enrichment than those of the other metals.
-
The mCd and ER values in all seasons for sites 3, 6, and 7 were the highest and classified as moderate-grade risk (mCd > 1.5; ER > 150), where the average contribution of Cd to ER was about 80%, followed by Ni (8%) and Pb (5%).
-
The ƩTU and TRI values at the anthropogenically influenced areas (sites 3, 4, 6, and 7) recorded values greater than 4 and 10, respectively. These indicate a moderate level of pollution. Ni and Cd contributed the most to the toxic risk indices (ƩTU and TRI).
-
The aquatic plants E. crassipes and C. demersum were found to have a high potential to accumulate heavy metals in their tissues, particularly in the anthropogenically impacted areas of the branch.
-
Cd, which was considered the major pollutant in the water and sediments of the Rosetta Branch, was able to accumulate in the tissues of E. crassipes and C. demersum in self-purification processes according to the BCF values. Consequently, these species could be considered as heavy metal bioindicators of Nile pollution.
Data Availability
The data can be available on request.
References
Abd El-Hady, H. H. (2014). Alternations in biochemical structures of phytoplankton in Aswan Reservoir and River Nile. Egypt. Journal of Biological and Environmental Sciences, 4(2), 68–80.
Abdelmageed, A. A., Abd Ellah, R. G., Abdel-Satar, A. M., et al. (2022). Evaluation of the ecological health and food chain on the shores of four River Nile Islands, Egypt. Environmental Monitoring and Assessment, 149(4), 309. https://doi.org/10.1007/s10661-022-09959-w
Abdel-Satar, A. M., & Geneid, Y. A. (2009). Evaluation of heavy metal status in ecosystem of Lake Manzalah, Egypt. Global Journal of Environmental Research, 3(3), 194–204.
Abdel-Satar, A. M., Ali, M. H. H., & Goher, M. E. (2017a). Indices of water quality and metal pollution of Nile River, Egypt. Egyptian Journal of Aquatic Research, 43, 21–29. https://doi.org/10.1016/j.ejar.2016.12.006
Abdel-Satar, A. M., Ali, M. H. H., & Goher, M. E. (2017b). Distribution and speciation of Fe, Mn, Zn, Cu, Pb and P in surface sediments of Mariut Lake, Egypt. Oceanological and Hydrobiological Studies, 46, 154–167. https://doi.org/10.1515/ohs-2017-0016
Abdel-Satar, A. M., Belal, D. M., Salem, S. G., Abdelmageed, A. A., Abdo, M. H., Abdel Gawad, S. S., & Al-Afify, A. D. G. (2022). Benthic diatoms and macroinvertebrates status with relevant to sediment quality of islands shores in the Nile River, Egypt. Rendiconti Lincei. Scienze Fisiche e Naturali. https://doi.org/10.1007/s12210-022-01051-2
Abou El-Anwar, E., Salman, S., Asmoay, A., & Elnazer, A. (2021). Geochemical, mineralogical and pollution assessment of River Nile sediments at Assiut Governorate, Egypt. Journal of African Earth Sciences, 180, 104227.
Abrahim, G., & Parker, R. (2008). Heavy metal contaminants in Tamaki estuary: Impact of city development and growth, Auckland, New Zealand. Environmental Geology, 42, 883–890.
Aktar, W. M., Paramasivam, M., Ganguly, M., Purkait, S., & Sengupta, D. (2010). Assessment and occurrence of various heavy metals in surface water of Ganga River around Kolkata: A study for toxicity and ecological impact. Environmental Monitoring and Assessment, 160, 207–213. https://doi.org/10.1007/s10661-008-0688-5
Al-Afify, A. D. G., & Abdel-Satar, A. M. (2020). Risk assessment of heavy metal pollution in water, sediment and plants in the Nile River in the Cairo region, Egypt. Oceanological and Hydrobiological Studies, 49, 1–12. https://doi.org/10.1515/ohs020-0001
Al-Afify, A. D. G., Othman, A. A., & Ramadan, M. A. (2018). Characterization of chemical and microbiological quality of Nile River surface water at Cairo (Egypt). Rendiconti Lincei. Scienze Fisiche e Naturali, 29, 725–736. https://doi.org/10.1007/s12210-018-0721-8
Algül, F., & Beyhan, M. (2020). Concentrations and sources of heavy metals in shallow sediments in Lake Bafa, Turkey. Scientific Reports, 10, 11782. https://doi.org/10.1038/s41598-020-68833-2
Ali, E. M., Shabaan-Dessouki, S. A., Soliman, A. I., & El-Shenawy, A. S. (2014). Characterization of chemical water quality in the Nile River, Egypt. International Journal of Pure and Applied Bioscience, 2(3), 35–53.
Ali, H., Khan, E., & Ilahi, I. (2019). Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. Journal of Chemistry, 2019(4), 1–14. https://doi.org/10.1155/2019/6730305
Ali, M., Rahman, S., Islam, S., et al. (2022). Distribution of heavy metals in water and sediment of an urban river in a developing country: A probabilistic risk assessment. International Journal of Sediment Research, 37, 173–187. https://doi.org/10.1016/j.ijsrc.2021.09.002
APHA (American Public Health Association), (2005). Standard methods for examination of water and wastewater (21st edn.). Standard methods is a joint publication of the American Public Health Association (APHA), the American Water Works Association (AWWA), and the Water Environment Federation (WEF). Washington, DC.
APRP, (2002). Survey of Nile system pollution sources. Cairo, Egypt. Ministry of water resource and irrigation, agricultural policy program – Water policy program report number 64.
Authman, M. M. N., El-Kasheif, M. A., & Shalloof, K. A. S. (2009). Evaluation and management of the fisheries of Tilapia species in Damietta Branch of the River Nile, Egypt. World Journal of Fish and Marine Sciences, 1, 167–184.
Avigliano, E., & Schenone, N. F. (2015). Human health risk assessment and environmental distribution of trace elements, glyphosate, fecal coliform and total coliform in Atlantic Rainforest mountain rivers (South America). Microchemical Journal, 122, 149–158. https://doi.org/10.1016/j.microc.2015.05.004
Backman, B., Bodis, D., Lahermo, P., & Rapant, S. (1997). Application of a groundwater contamination index in Finland and Slovakia. Environmental Geology, 36(1–2), 55–64.
Bastami, K. D., Afkhami, M., Mohammadizadeh, M., Ehsanpour, M., Chambari, S., Aghaei, S., Esmaeilzadeh, M., Neyestani, M. R., Lagzaee, F., & Baniamam, M. (2015). Bioaccumulation and ecological risk assessment of heavy metals in the sediments and mullet Liza klunzingeri in the northern part of the Persian Gulf. Marine Pollution Bulletin, 94, 329–334. https://doi.org/10.1016/j.marpolbul.2015.01.019
Caerio, S., Costa, M. H., Ramos, T. B., Fernandes, F., Silverira, N., Coimbra, A., & Painho, M. (2005). Assessing heavy metal contamination in Sado estuary sediment: An index analysis approach. Ecological Indicators, 5(1), 155–169.
Dey, M., Akter, A., Islam, S., Dey, S. C., et al. (2021). Assessment of contamination level, pollution risk and source apportionment of heavy metals in the Halda River water, Bangladesh. Heliyon, 7, e08625.
Eissa, F., Al-Sisi, M., & Ghanem, K. (2022). Occurrence and ecotoxicological risk assessment of pesticides in sediments of the Rosetta branch, Nile River, Egypt. Journal of Environmental Sciences, 118, 21–31. https://doi.org/10.1016/j.jes.2021.08.047
EPA (Environmental Protection Agency), (2006). Region III BTAG freshwater sediment screening benchmarks 8/2006.
EWQS (Egyptian drinking water quality standards), (2007). Ministry of Health, Population Decision number (458).
Gao, L., Wang, Z., Li, S., & Chen, J. (2018). Bioavailability and toxicity of trace metals (Cd, Cr, Cu, Ni, and Zn) in sediment cores from the Shima River, South China. Chemosphere, 192, 31–42. https://doi.org/10.1016/j.chemosphere.2017.10.110
Hadi, M. P., Fadlillah, L. N., Sih Tifani, A. R., & Ramdan, V. K. (2019). Heavy metal pollution and water quality assessment in Belik River Yogyakarta. IOP Conference Series: Earth and Environmental Science, 256, 012014.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14, 975–1001.
Hakanson, L. (1988). Metal monitoring in coastal environments. In U. Seeliger, L. D. Lacerda, & S. R. Patchineelam (Eds.), Metals in Coastal Environments of Latin America (pp. 240–257). Springer Verlag.
Harguinteguy, C. A., Cirelli, A. F., & Pignata, M. L. (2014). Heavy metal accumulation in leaves of aquatic plant Stuckenia filiformis and its relationship with sediment and water in the Suquía River (Argentina). Microchemical Journal, 114, 111–118.
Idodo-Umeh, G., & Oronsaye, J. A. O. (2006). Heavy metal pollution of the sediments from Eriora River in Olomoro town, Niger Delta, Nigeria. Journal of Sustainable Agricultural Resources, 21, 74–81.
Jiao, C. Z., Li, H., Song, M., & Wang, L. (2018). Ecological risk assessment of heavy metals in water and sediment of the Pearl River estuary. IOP Conference Series: Materials Science and Engineering, 394, 052055. https://doi.org/10.1088/1757-899X/394/5/052055
Kouadia, L., & Trefry, J. H. (1987). Saline trace metal contamination in the Ivory. Air Water Soil Pollution, 32, 145–154.
Kowalska, J. B., Mazurek, R., Gasiorek, M., & Zalesk, T. (2018). Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination–A review. Environmental Geochemistry and Health, 40, 2395–2420.
Lipy, E. P., Hakim, M., Mohanta, L. C., et al. (2021). Assessment of heavy metal concentration in water, sediment and common fish species of Dhaleshwari River in Bangladesh and their health implications. Biological Trace Element Research, 199, 4295–4307. https://doi.org/10.1007/s12011-020-02552-7
Loucks, D. P., & van Beek, E. (2017). Water resources planning and management: An overview. In: Water Resource Systems Planning and Management. Springer, Cham.
Lu, G., Wang, B., Zhang, C., Li, S., Wen, J., Lu, G., Zhu, C., & Zhou, Y. (2018). Heavy metals contamination and accumulation in submerged macrophytes in an urban river in China. International Journal of Phytoremediation, 20(8), 839–846. https://doi.org/10.1080/15226514.2018.1438354
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31. https://doi.org/10.1007/s002440010075
Masresha, A. E., Skipperud, L., Rosseland, B. O., Zinabu, G. M., Meland, S., & Salbu, B. (2021). Bioaccumulation of trace elements in liver and kidney of fish species from three freshwater lakes in the Ethiopian Rift Valley. Environmental Monitoring and Assessment, 193, 329. https://doi.org/10.1007/s10661-021-09083-1
Müller, G. (1969). Index of geo-accumulation in sediments of the Rhine River. GeoJournal, 2(3), 108–118.
Othman, A. A., Al-Afify, A. D. G., Abdel-Satar, A. M., & Ramadan, M. F. (2021). Quality assessment of surface water using the Nile Chemical Pollution Index (NCPI) and microbiological pollution of the Rosetta Branch (Nile River, Egypt). African Journal of Aquatic Science, 46(2), 129–141. https://doi.org/10.2989/16085914.2020.1807898
Paronda, G. R. A., David, C. P. C., & Apodaca, D. C. (2019). River flow pattern and heavy metals concentrations in Pasig River, Philippines as affected by varying seasons and astronomical tides. IOP Conference Series: Earth and Environmental Science, 344, 012049. https://doi.org/10.1088/1755-1315/344/1/012049
Pedersen, F., Bjørnestad, E., Andersen, H. V., Kjølholt, J., & Poll, C. (1998). Characterization of sediments from Copenhagen Harbour by use of biotests. Water Science & Technology, 37, 233–240.
Prasad, B., & Bose, J. M. (2001). Evaluation of heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environmental Geology, 41, 183–188.
Saison, C., Schwartz, C., & Morel, J. L. (2004). Hyperaccumulation of metals by Thlaspi caerulescens as affected by root development and Cd-Zn/Ca-Mg interaction. International Journal of Phytoremediation, 6(1), 49–61.
Sani, A., Idris, K. M., Abdullahi, B. A., & Darma, A. I. (2022). Bioaccumulation and health risks of some heavy metals in Oreochromis niloticus, sediment and water of Challawa River, Kano, Northwestern Nigeria. Environmental Advances, 7, 100172. https://doi.org/10.1016/j.envadv.2022.100172
Sun, L., & Zheng, L. (2018). The ecological risk assessment of heavy metals in the Kuihe River basin (Xuzhou section) and the characteristics of plant enrichment. IOP Conf. Series: Earth and Environmental Science, 108, 042026.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgolander Meeresunters, 33, 566–575. https://doi.org/10.1007/BF02414780
Tshithukhe, G., Motitsoe, S. N., & Hill, M. P. (2021). Heavy metals assimilation by native and non-native aquatic macrophyte species: A case study of a river in the Eastern Cape Province of South Africa. Plants, 10, 2676. https://doi.org/10.3390/plants10122676
Turkmen, A., Turkmen, M., Tepe, Y., Mazlum, Y., & Oymael, S. (2006). Metal concentrations in Blue Crab (Callinectes sapidus) and Mullet (Mugil cephalus) in Iskenderun Bay, Northern East Mediterranean, Turkey. Bulletin of Environmental Contamination and Toxicology, 77, 186–193. https://doi.org/10.1007/s00128-006-1049-0
USEPA (US. Environmental Protection Agency), (1993). Office of Water Policy and Technical Guidance on Interpretation and Implementation of Aquatic Life Metals Criteria. Office of Water Regulations and Standards Division: Washington, DC, USA
WHO (World Health Organization), (2017). Guidelines for drinking-water quality: Fourth edition incorporating the first addendum. Geneva: World Health Organization, Licence: CC BY-NC-SA 3.0 IGO.
WHO/FAO, (2007). Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission 13th Session. Report of the Thirty-Eight Session of the Codex Committee on Food Hygiene, Houston, United States of America, ALINORM 07/30/13
Yacoub, A. M., Mahmoud, S. A., & Abdel-Satar, A. M. (2021). Accumulation of heavy metals in tilapia fish species and related histopathological changes in muscles, gills and liver of Oreochromis niloticus occurring in the area of Qahr El-Bahr, Lake Al-Manzalah, Egypt. Oceanological and Hydrobiological Studies, 50, 1–15. https://doi.org/10.2478/oandhs-2021-0001
Yahaya, M. I., Jacob, A. G., Agbendeh, Z. M., Akpan, G. P., & Kwasara, A. A. (2012). Seasonal potential toxic metals contents of Yauri River bottom sediments: North western Nigeria. Journal of Environmental Chemistry and Ecotoxicology, 4(12), 212–221.
Yu, H., Lin, M., Peng, W., & He, C. (2022). Seasonal changes of heavy metals and health risk assessment based on Monte Carlo simulation in alternate water sources of the Xinbian River in Suzhou City, Huaibei Plain, China. Ecotoxicology and Environmental Safety, 236, 113445. https://doi.org/10.1016/j.ecoenv.2022.113445
Zhao, Q., Ding, S., Hong, Z., et al. (2021). Impacts of water-sediment regulation on spatial-temporal variations of heavy metals in riparian sediments along the middle and lower reaches of the Yellow River. Ecotoxicology and Environmental Safety, 227, 112943. https://doi.org/10.1016/j.ecoenv.2021.112943
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Afify DG Al-Afify: conceptualization, collection of samples, heavy metals analysis, data curation, and writing the original draft. Amaal M Abdel-Satar: chemical data curation, writing, review, and final editing for the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Afify, A.D.G., Abdel-Satar, A.M. Heavy Metal Contamination of the River Nile Environment, Rosetta Branch, Egypt. Water Air Soil Pollut 233, 302 (2022). https://doi.org/10.1007/s11270-022-05759-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-022-05759-7