Abstract
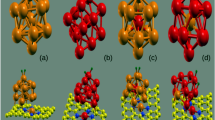
Hydrogen gas is a promising renewable energy source. The hydrogen storage performance of two differently modified graphene surfaces, particularly Cu-doped and Cu-decorated circumcoronene (CC), is investigated using density functional theory, 6-311G* basis set and Bader's quantum theory of atoms in molecules (QTAIM). It is found that the Cu-doped CC is able to bind three H2 molecules on one Cu atom, while the Cu-decorated CC is able to bind up to five H2 molecules on one Cu atom. Changes in the topology of charge density upon the H2 adsorption are evaluated under the formalism of QTAIM analysis. The QTAIM analysis of bond critical points as well as the density of states analysis show that the interaction between Cu and adsorbed H2 molecules can be considered as a physisorption (a van der Waals type interaction). Overall, the results presented in this study point out that the Cu-decorated graphene surfaces are more suitable potential candidates for hydrogen storage than the Cu-doped ones. Furthermore, the inclusion of diffuse functions in the basis set is critically considered.







Similar content being viewed by others
References
Novoselov KS, Geim AK, Morozov SV et al (2004) Electric field effect in atomically thin carbon films. Science 306:666–669. https://doi.org/10.1126/science.1102896
Pykal M, Jurecka P, Karlicky F, Otyepka M (2016) Modelling of graphene functionalization. Phys Chem Chem Phys 18:6351–6372. https://doi.org/10.1039/C5CP03599F
Schedin F, Geim A, Morozov SV et al (2007) Detection of individual gas molecules adsorbed on graphene. Nat Mater 6:652–655. https://doi.org/10.1038/nmat1967
Yang W, Ratinac KR, Ringer SR et al (2010) Carbon nanomaterials in biosensors: should you use nanotubes or graphene. Angew Chemie Int Ed 49:2114–2138. https://doi.org/10.1002/anie.200903463
Liu Y, Dong X, Chen P (2012) Biological and chemical sensors based on graphene materials. Chem Soc Rev 41:2283–2307. https://doi.org/10.1039/c1cs15270j
Rodrigo D, Limaj O, Janner D et al (2015) Mid-infrared plasmonic biosensing with graphene. Science 349:165–168. https://doi.org/10.1126/science.aab2051
Ambrosi A, Chua CK, Bonanni A, Pumera M (2014) Electrochemistry of graphene and related materials. Chem Rev 114:7150–7188. https://doi.org/10.1021/cr500023c
Lv R, Terrones M (2012) Towards new graphene materials: Doped graphene sheets and nanoribbons. Mater Lett 78:209–218. https://doi.org/10.1016/j.matlet.2012.04.033
Zhou M, Lu Y-H, Cai Y-Q et al (2011) Adsorption of gas molecules on transition metal embedded graphene: a search for high-performance graphene-based catalysts and gas sensors. Nanotechnology 22:385502. https://doi.org/10.1088/0957-4484/22/38/385502
Düzenli D (2016) A comparative density functional study of hydrogen peroxide adsorption and activation on the graphene surface doped with N, B, S, Pd, Pt, Au, Ag and Cu Atoms. J Phys Chem C 120:20149–20157. https://doi.org/10.1021/acs.jpcc.6b06131
Wu P, Du P, Zhang H, Cai C (2013) Microscopic effects of the bonding configuration of nitrogen-doped graphene on its reactivity toward hydrogen peroxide reduction reaction. Phys Chem Chem Phys 15:6920. https://doi.org/10.1039/c3cp50900a
Liu S, Huang S (2017) Theoretical insights into the activation of O2 by Pt single atom and Pt4 nanocluster on functionalized graphene support: Critical role of Pt positive polarized charges. Carbon N Y 115:11–17. https://doi.org/10.1016/j.carbon.2016.12.094
Mohammadi-Manesh E, Vaezzadeh M, Saeidi M (2015a) Cu- and CuO-decorated graphene as a nanosensor for H2S detection at room temperature. Surf Sci 636:36–41. https://doi.org/10.1016/j.susc.2015.02.002
Mohammadi-Manesh E, Vaezzadeh M, Saeidi M (2015b) Theoretical study on electronic structure and electrical conductance at room temperature of Cu2O-GS nanosensors and detection of H2S gas. Comput Mater Sci 97:181–185. https://doi.org/10.1016/j.commatsci.2014.10.043
Maaghoul Z, Fazileh F, Kakemam J (2015) A DFT study of formaldehyde adsorption on functionalized graphene nanoribbons. Phys E Low Dimens Syst Nanostructures 66:176–180. https://doi.org/10.1016/j.physe.2014.08.015
Rad AS (2015) First principles study of Al-doped graphene as nanostructure adsorbent for NO 2 and N 2 O : DFT calculations. Appl Surf Sci j 357:1217–1224
Wanno B, Tabtimsai C (2014) A DFT investigation of CO adsorption on VIIIB transition metal-doped graphene sheets. Superlattices Microstruct 67:110–117. https://doi.org/10.1016/j.spmi.2013.12.025
Dai J, Yuan J, Giannozzi P (2009) Gas adsorption on graphene doped with B, N, Al and S: s theoretical study. Appl Phys Lett 95:232105. https://doi.org/10.1063/1.3272008
Zhou L, Shen F, Tian X et al (2013) Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale 5:1564. https://doi.org/10.1039/c2nr33164k
Malček M, Cordeiro MNDS (2018) A DFT and QTAIM study of the adsorption of organic molecules over the copper-doped coronene and circumcoronene. Phys E Low-Dimens Syst Nanostructures 95:59–70. https://doi.org/10.1016/j.physe.2017.09.004
Janani K, John Thiruvadigal D (2017) Chemical functionalization and edge doping of zigzag graphene nanoribbon with L-(+)-leucine and group IB elements—A DFT study. Appl Surf Sci 418:406–413. https://doi.org/10.1016/j.apsusc.2017.02.192
Malček M, Bučinský L, Teixeira F, Cordeiro MNDS (2018) Detection of simple inorganic and organic molecules over Cu-decorated circumcoronene: a combined DFT and QTAIM study. Phys Chem Chem Phys 20:16021–16032. https://doi.org/10.1039/c8cp02035c
Bosch-Navarro C, Rourke JP, Wilson NR (2016) Controlled electrochemical and electroless deposition of noble metal nanoparticles on graphene. RSC Adv 6:73790–73796. https://doi.org/10.1039/C6RA14836K
Feng X, Lv P, Sun W et al (2017) Reduced graphene oxide-supported Cu nanoparticles for the selective oxidation of benzyl alcohol to aldehyde with molecular oxygen. Catal Commun 99:105–109. https://doi.org/10.1016/j.catcom.2017.05.013
Bayev VG, Fedotova JA, Kasiuk JV et al (2018) CVD graphene sheets electrochemically decorated with “core-shell” Co/CoO nanoparticles. Appl Surf Sci 440:1252–1260. https://doi.org/10.1016/j.apsusc.2018.01.245
Durán GM, Benavidez TE, Contento AM et al (2017) Analysis of penicillamine using Cu-modified graphene quantum dots synthesized from uric acid as single precursor. J Pharm Anal 7:324–331. https://doi.org/10.1016/j.jpha.2017.07.002
Lonkar SP, Deshmukh YS, Abdala AA (2015) Recent advances in chemical modifications of graphene. Nano Res 8:1039–1074. https://doi.org/10.1007/s12274-014-0622-9
Lin L, Zhou W, Gao R et al (2017) Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nat Publ Gr 544:80–83. https://doi.org/10.1038/nature21672
Xiang C, Li A, Yang S et al (2019) Enhanced hydrogen storage performance of graphene nano fl akes doped with Cr atoms : a DFT. RSC Adv 9:25690–25696. https://doi.org/10.1039/c9ra04589a
Tabtimsai C, Rakrai W, Wanno B (2017) Hydrogen adsorption on graphene sheets doped with group 8B transition metal : a DFT investigation. Vaccum 139:101–108. https://doi.org/10.1016/j.vacuum.2017.02.013
Zhou Y, Chu W, Jing F et al (2017) Applied Surface Science Enhanced hydrogen storage on Li-doped defective graphene with B substitution : a DFT study . Appl Surf Sci 410:166–176. https://doi.org/10.1016/j.apsusc.2017.03.057
Wang L, Chen X, Du H et al (2018) First-principles investigation on hydrogen storage performance of Li, Na and K decorated borophene. Appl Surf Sci 427:1030–1037. https://doi.org/10.1016/j.apsusc.2017.08.126
Yang S, Lan Z, Xu H et al (2018) A first-principles study on hydrogen sensing properties of pristine and Mo-doped graphene. Int J Nanotechnol 2018:1–5. https://doi.org/10.1155/2018/2031805
Faye O, Eduok U, Szpunar J et al (2017) Hydrogen storage on bare Cu atom and Cu-functionalized boron-doped graphene: a first principles study. Int J Hydrog Energy 42:4233–4243. https://doi.org/10.1016/j.ijhydene.2016.10.031
Faye O, Szpunar JA, Szpunar B, Chedikh A (2017) Hydrogen adsorption and storage on Palladium—functionalized graphene with NH-dopant : a first principles calculation. Appl Surf Sci 392:362–374. https://doi.org/10.1016/j.apsusc.2016.09.032
Ruiz ME, Garcia-Prieto J, Novaro O (1984) Theoretical studies of photoexcited state Cu atom reactions. I. excited state responsible for H2 capture. J Chem Phys 80:1529–1534. https://doi.org/10.1063/1.446902
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford university press, Oxford
Colherinhas G, Fileti EE, Chaban VV (2015a) The band gap of graphene is efficiently tuned by monovalent ions. J Phys Chem Lett 6:302–307. https://doi.org/10.1021/jz502601z
Colherinhas G, Fileti EE, Chaban VV (2015b) Can inorganic salts tune electronic properties. Phys Chem Chem Phys 17:17413–17420. https://doi.org/10.1039/C5CP02083B
Colherinhas G, Fileti EE, Chaban VV (2016) Potential energy surface of excited semiconductors: graphene quantum dot and BODIPY. Chem Phys 474:1–6. https://doi.org/10.1016/j.chemphys.2016.05.011
Zhu C, Yang G (2016) Insights from the adsorption of halide ions on graphene materials. Chem Phys Chem 17:2482–2488. https://doi.org/10.1002/cphc.201600271
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Development of the colic-salvetti correlation-energy formula into a functional of the electron D. Phys Rev B 37:785–789
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Vosko SH, Wilk L, Nusair M (1980) Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can J Phys 58:1200–1211. https://doi.org/10.1139/p80-159
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. a basis set for correlated wave functions. J Chem Phys 72:650–654
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. second row atoms, Z=11–18. J Chem Phys 72:5639–5648
Wachters AJH (1970) Gaussian basis set for molecular wavefunctions containing third-row atoms. J Chem Phys 52:1033–1036
Sousa SF, Fernandes PA, Ramos MJ (2007) General performance of density functionals. J Phys Chem A 111:10439–10452. https://doi.org/10.1021/jp0734474
Ramalho JPP, Gomes JRB, Illas F (2013) Accounting for van der Waals interactions between adsorbates and surfaces in density functional theory based calculations: selected examples. RSC Adv 3:13085–13100. https://doi.org/10.1039/c3ra40713f
Grimme S, Hansen A, Brandenburg JG, Bannwarth C (2016) Dispersion-corrected mean-field electronic structure methods. Chem Rev 116:5105–5154. https://doi.org/10.1021/acs.chemrev.5b00533
Mollenhauer D, Brieger C, Voloshina E, Paulus B (2015) Performance of dispersion-corrected DFT for the weak interaction between aromatic molecules and extended carbon-based systems. J Phys Chem C 119:1898–1904. https://doi.org/10.1021/jp5113312
Le D, Kara A, Schroder E et al (2012) Physisorption of nucleobases on graphene: a comparative van der Waals study. J Phys Condens Matter 24:424210. https://doi.org/10.1088/0953-8984/24/42/424210
Frisch MJ, Trucks GW, Schlegel HB, et al. (2009) Gaussian 09, Revision D.01
Keith TA AIMAll, version 14.04.17; TK Gristmill software: Overland Park, KS, 2014 (aim.tkgristmill.com)
O’Boyle NM, Tenderholt AL, Langner KM (2008) cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845. https://doi.org/10.1002/jcc.20823
Flükiger P, Lüthi HP, Sortmann S, Weber J “MOLEKEL 4.3.” Swiss center for scientific computing: Manno, Switzerland 2002
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function—augmented basis sets for anion calculations. III.* The 3–21 + G basis set for first-row. J Comp Chem 4:294–301. https://doi.org/10.1002/jcc.540040303
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Bacskay GB (1952) A quadritically convergent hartree-fock (QC-SCF) method. Application to open shell orbital optimization and coupled perturbed hartree-fock calculations. Chem Phys 65:383–396. https://doi.org/10.1016/0301-0104(82)85211-7
Luna-Garcia H, Ramirez-Solis A, Castillo S (2002) Ab initio studies of the reactions of Cu (2 S, 2 D and 2 P) with SiH 4 and GeH 4. J Chem Phys 116:928–935. https://doi.org/10.1063/1.1427713
Bhagi-Damodaran A, Michael MA, Zhu Q et al (2017) Why copper is preferred over iron for oxygen activation and reduction in haem-copper oxidases. Nat Chem 9:257–263. https://doi.org/10.1038/nchem.2643
López CS, de Lera ÁR (2011) Bond ellipticity as a measure of electron delocalization in structure and reactivity. Curr Org Chem 15:3576–3593. https://doi.org/10.2174/138527211797636228
Bader RFW, Stephens ME (1975) Spatial localization of the electronic pair and number distributions in molecules. J Am Chem Soc 97:7391–7399. https://doi.org/10.1021/ja00859a001
Afonin AV, Vashchenko AV, Sigalov MV (2016) Estimating the energy of intramolecular hydrogen bonds from 1 H NMR and QTAIM calculations. Org Biomol Chem 14:11199–11211. https://doi.org/10.1039/C6OB01604A
Arslancan S, Herrera B, Lamsabhi AM (2020) On the nature of the interaction of copper hydride and halide with substituted ethylene and acetylene. J Mol Model 26:61. https://doi.org/10.1007/s00894-020-4320-0
Acknowledgements
This work received financial support from Slovak Grant Agencies APVV (contracts No. APVV-15-0079, APVV-15-0053, APVV-19-0024 and APVV-19-0087) and VEGA (contracts No. 1/0139/20 and 1/0466/18). We are also grateful to the HPC center at the Slovak university of technology in Bratislava, which is a part of the Slovak infrastructure of high performance computing (SIVVP project, ITMS code 26230120002, funded by the European region development funds) for the computational time and resources made available. To all financing sources the authors are greatly indebted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as part of the topical collection of articles from the 17th edition of the Central European Symposium on Theoretical Chemistry (CESTC 2019) in Austria
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malček, M., Bučinský, L. On the hydrogen storage performance of Cu-doped and Cu-decorated graphene quantum dots: a computational study. Theor Chem Acc 139, 167 (2020). https://doi.org/10.1007/s00214-020-02680-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-020-02680-2