Abstract
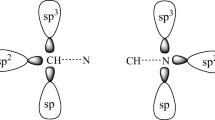
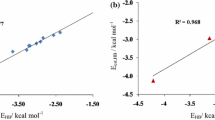
Ab initio calculations at the MP2/aug-cc-pVTZ level have been performed to study the cooperativity of hydrogen bonds in homoclusters (HNC–HNC–HNC and HNC–HNC–HNC–HNC) and heteroclusters (H3N–HNC–HNC and H3N–HNC–HNC–HNC). The cooperative energies in the HNC–HNC–HNC and H3N–HNC–HNC trimers are –2.05 and –2.56 kcal/mol, respectively. The result shows that the cooperativity in the heterotrimer is larger than that in the homotrimer. A similar result also happens in the tetramers. The energy decomposition scheme indicates that orbital interaction is a major contribution to the cooperative energy of N···HN hydrogen bond, whereas the electrostatic and orbital interactions to that of C···HN hydrogen bond. The effect of HNC chain length on the strength of N···HN hydrogen bond has also been considered at the MP2/aug-cc-pVDZ level. It is indicated that the interaction energy of N···HN hydrogen bond trends to be a fixed value when the HNC number tends to be infinite, and the strength of N···HN hydrogen bond is regulated mainly through the electrostatic and polarization interactions although the charge transfer interaction also has an effect on it.



Similar content being viewed by others
References
Jeffrey GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Cabaleiro-Lago EM, Rios MA (1999) J Phys Chem A 103:6468
Gaw JF, Yamaguchl Y, Vincent MA, Schaefer HF (1984) J Am Chem Soc 106:3133
Andrews L, Johnson GL (1983) J Chem Phys 79:3670
Ayers GP, Pullin ADE (1976) Spectrochim Acta A 32:1641
Engdahl A, Nelander B (1986) J Phys Chem 90:6118
Hannachi Y, Schriver L, Schriver A, Perchard JP (1989) Chem Phys 135:285
Hannachi Y, Silvi B, Bouteiller JY (1992) Chem Phys 97:1911
Kreissler M, Lavialle L, Boggio-Pasqua M, Hannachi Y (1998) J Mol Struct 542:55
Milligan DE, Jacox ME (1967) J Chem Phys 47:278
Brown RD, Godfrey PD, Storey JWV, Clark FO (1976) Nature 262:672
Liebman SA, Pesce-Rodriguez RAP, Mattews CN (1994) Adv Space Res 15:71
Wang ZX, Zhang JC, Wu JY, Cao WL (2007) J Mol Struct 806:239
Wang BQ, Li ZR, Wu D, Hao XY, Li RJ, Sun CC (2004) J Phys Chem A 108:2464
Meot-Ner M, Speller CV (1989) J Phys Chem 933:663
Wang CX, Zhang JC, Cao WL (2007) Chem J Chin Univ Chin 2:320
Alkorta I, Rozas I, Elguero J (1998) Theor Chem Acc 99:116
Li QZ, An XL, Luan F, Li WZ, Gong BA, Cheng JB, Sun JZ (2008) J Chem Phys 128:154102
Boys SF, Bernardi F (1970) Mol Phys 19:553
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2003) Gaussian 03, Revision E.03. Gaussian, Inc., Pittsburgh, PA
ADF2008.01, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. http://www.scm.com
Cubero E, Orozco M, Hobza P, Luque FJ (1999) J Phys Chem A 103:6394
Bian L (2003) J Phys Chem A 107:11517
Li QZ, An XL, Gong BA, Cheng JB (2007) J Phys Chem A 111:10166
Li QZ, Liu ZB, Cheng JB, Li WZ, Gong BA, Sun JZ (2009) J Mol Struct 896:112
Kar T, Scheiner S (2004) J Phys Chem A 108:9161
King BF, Weinhold F (1995) J Chem Phys 103:333
Li QZ, Hu T, An XL, Gong BA, Cheng JB (2008) Chemphyschem 9:1942
Guerra CF, van der Wijst T, Bickelhaupt FM (2005) Struct Chem 16:211
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 20973149) and in part by open project of State Key Laboratory of Supramolecular Structure and Materials (SKLSSM200909) from Jilin University, China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gong, B., Jing, B., Li, Q. et al. Ab initio study of the cooperativity between NH···N and NH···C hydrogen bonds in H3N–HNC–HNC complex. Theor Chem Acc 127, 303–309 (2010). https://doi.org/10.1007/s00214-009-0716-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0716-8