Abstract
Rationale
Whereas reward-modulatory opioid actions have been intensively studied in subcortical sites such as the nucleus accumbens (Acb), the role of cortical opioid transmission has received comparatively little attention.
Objectives
The objective of this study is to describe recent findings on the motivational actions of opioids in the prefrontal cortex (PFC), emphasizing studies of food motivation and ingestion. PFC-based opioid effects will be compared/contrasted to those elicited from the Acb, to glean possible common functional principles. Finally, the motivational effects of opioids will be placed within a network context involving the PFC, Acb, and hypothalamus.
Results
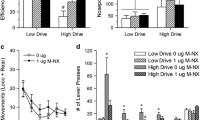
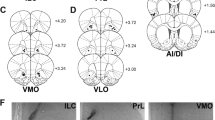
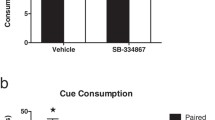
Mu-opioid receptor (μ-OR) stimulation in both the Acb and PFC induces eating and enhances food-seeking instrumental behaviors; μ-OR signaling also enhances taste reactivity within a highly circumscribed zone of medial Acb shell. In both the Acb and PFC, opioid-sensitive zones are aligned topographically with the sectors that project to feeding-modulatory zones of the hypothalamus and intact glutamate transmission in the lateral/perifornical (LH-PeF) hypothalamic areas is required for both Acb- and PFC-driven feeding. Conversely, opioid-mediated feeding responses elicited from the PFC are negatively modulated by AMPA signaling in the Acb shell.
Conclusions
Opioid signaling in the PFC engages functionally opposed PFC➔hypothalamus and PFC➔Acb circuits, which, respectively, drive and limit non-homeostatic feeding, producing a disorganized and “fragmented” pattern of impulsive food-seeking behaviors and hyperactivity. In addition, opioids act directly in the Acb to facilitate food motivation and taste hedonics. Further study of this cortico-striato-hypothalamic circuit, and incorporation of additional opioid-responsive telencephalic structures, could yield insights with translational relevance for eating disorders and obesity.

Similar content being viewed by others
References
Aberman JE, Salamone JD (1999) Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience 92:545–552
Apfelbaum M, Mandenoff A (1981) Naltrexone suppresses hyperphagia induced in the rat by a highly palatable diet. Pharmacol Biochem Behav 15:89–91
Bakshi VP, Kelley AE (1991) Dopaminergic regulation of feeding-behavior.1. Differential-effects of haloperidol microinfusion into 3 striatal subregions. Psychobiology 19:223–232
Bakshi VP, Kelley AE (1993) Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology 111:207–214
Baldo BA (2016) Prefrontal cortical opioids and dysregulated motivation: a network hypothesis. Trends Neurosci 39(6):366–377
Baldo BA, Kelley AE (2007) Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology 191:439–459
Baldo BA, Sadeghian K, Basso AM, Kelley AE (2002) Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res 137:165–177
Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE (2004) Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci 19:376–386
Baldo BA, Pratt WE, Will MJ, Hanlon EC, Bakshi VP, Cador M (2013) Principles of motivation revealed by the diverse functions of neuropharmacological and neuroanatomical substrates underlying feeding behavior. Neurosci Biobehav Rev 37:1985–1998
Baldo BA, Spencer RC, Sadeghian K, Mena JD (2016) GABA-mediated inactivation of medial prefrontal and Agranular insular cortex in the rat: contrasting effects on hunger- and palatability-driven feeding. Neuropsychopharmacology 41:960–970
Ball GF, Balthazart J (2008) How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of sexual behavior? Horm Behav 53:307–311 author reply 315-8
Barbano MF, Cador M (2006) Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology 31:1371–1381
Barbano MF, Cador M (2007) Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology 191:497–506
Berridge KC (1991) Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite 16:103–120
Berridge KC (2004) Motivation concepts in behavioral neuroscience. Physiol Behav 81:179–209
Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431
Berridge KC (2009) ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav 97:537–550
Berridge KC, Kringelbach ML (2015) Pleasure systems in the brain. Neuron 86:646–664
Berridge KC, Venier IL, Robinson TE (1989) Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci 103:36–45
Bindra D (1974) A motivational view of learning, performance, and behavior modification. Psychol Rev 81:199–213
Birrell JM, Brown VJ (2000) Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20:4320–4324
Blanchard RJ, Blanchard DC (1989) Antipredator defensive behaviors in a visible burrow system. J Comp Psychol 103:70–82
Blasio A, Steardo L, Sabino V, Cottone P (2014) Opioid system in the medial prefrontal cortex mediates binge-like eating. Addict Biol 19:652–662
Cabanac M, Duclaux R (1973) Olfactory-gustatory alliesthesia and food intake in humans. J Physiol Paris 66:113–135
Cambridge VC, Ziauddeen H, Nathan PJ, Subramaniam N, Dodds C, Chamberlain SR, Koch A, Maltby K, Skeggs AL, Napolitano A, Farooqi IS, Bullmore ET, Fletcher PC (2013) Neural and behavioral effects of a novel mu opioid receptor antagonist in binge-eating obese people. Biol Psychiatry 73:887–894
Cameron JD, Goldfield GS, Finlayson G, Blundell JE, Doucet E (2014) Fasting for 24 hours heightens reward from food and food-related cues. PLoS One 9:e85970
Clarke SN, Parker LA (1995) Morphine-induced modification of quinine palatability: effects of multiple morphine-quinine trials. Pharmacol Biochem Behav 51:505–508
Cooper SJ (1983) Effects of opiate agonists and antagonists on fluid intake and saccharin choice in the rat. Neuropharmacology 22:323–328
Cooper SJ, Turkish S (1989) Effects of naltrexone on food preference and concurrent behavioral responses in food-deprived rats. Pharmacol Biochem Behav 33:17–20
Craig W (1917) Appetites and aversions as constituents of instincts. Proc Natl Acad Sci U S A 3:685–688
Curley AA, Lewis DA (2012) Cortical basket cell dysfunction in schizophrenia. J Physiol 590:715–724
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784
Davis JD, Smith GP (1992) Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106:217–228
DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC (2012) Enkephalin surges in dorsal neostriatum as a signal to eat. Curr Biol 22:1918–1924
Dong D, Wang Y, Jackson T, Chen S, Wang Y, Zhou F, Chen H (2016) Impulse control and restrained eating among young women: Evidence for compensatory cortical activation during a chocolate-specific delayed discounting task. Appetite
Doyle TG, Berridge KC, Gosnell BA (1993) Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav 46:745–749
Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA (1992) Taste responses and preferences for sweet high-fat foods: evidence for opioid involvement. Physiol Behav 51:371–379
Dukas R (2002) Behavioural and ecological consequences of limited attention. Philos Trans R Soc Lond Ser B Biol Sci 357:1539–1547
Eddy MC, Todd TP, Bouton ME, Green JT (2016) Medial prefrontal cortex involvement in the expression of extinction and ABA renewal of instrumental behavior for a food reinforcer. Neurobiol Learn Mem 128:33–39
Evans KR, Vaccarino FJ (1990) Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev 14:9–22
Fantino M, Hosotte J, Apfelbaum M (1986) An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Phys 251:R91–R96
Ferezou I, Hill EL, Cauli B, Gibelin N, Kaneko T, Rossier J, Lambolez B (2007) Extensive overlap of mu-opioid and nicotinic sensitivity in cortical interneurons. Cereb Cortex 17:1948–1957
Fields HL, Margolis EB (2015) Understanding opioid reward. Trends Neurosci 38:217–225
Floresco SB, Block AE, Tse MT (2008) Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res 190:85–96
Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R (2001) Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol 432:307–328
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005) Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177
Giraudo SQ, Grace MK, Welch CC, Billington CJ, Levine AS (1993) Naloxone’s anorectic effect is dependent upon the relative palatability of food. Pharmacol Biochem Behav 46:917–921
Giraudo SQ, Kotz CM, Billington CJ, Levine AS (1998) Association between the amygdala and nucleus of the solitary tract in mu-opioid induced feeding in the rat. Brain Res 802:184–188
Glass MJ, Grace M, Cleary JP, Billington CJ, Levine AS (1996) Potency of naloxone’s anorectic effect in rats is dependent on diet preference. Am J Phys 271:R217–R221
Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML, Dannals RF, Frost JJ (2008) Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology 200:475–486
Grill HJ, Norgren R (1978) The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143:263–279
Groenewegen HJ, Berendse HW, Haber SN (1993) Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience 57:113–142
Haber SN, Groenewegen HJ, Grove EA, Nauta WJ (1985) Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol 235:322–335
Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN (2016) Circuit-based Corticostriatal homologies between rat and primate. Biol Psychiatry 80:509–521
Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C (1991) Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41:89–125
Ikeda H, Akiyama G, Fujii Y, Minowa R, Koshikawa N, Cools AR (2003) Role of AMPA and NMDA receptors in the nucleus accumbens shell in turning behaviour of rats: interaction with dopamine receptors. Neuropharmacology 44:81–87
Ikemoto S, Panksepp J (1999) The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev 31:6–41
Jezzini A, Mazzucato L, La Camera G, Fontanini A (2013) Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. J Neurosci 33:18966–18978
Karhunen LJ, Vanninen EJ, Kuikka JT, Lappalainen RI, Tiihonen J, Uusitupa MI (2000) Regional cerebral blood flow during exposure to food in obese binge eating women. Psychiatry Res 99:29–42
Kim SW, Grant JE, Adson DE, Shin YC (2001) Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry 49:914–921
Kim EM, Quinn JG, Levine AS, O’Hare E (2004) A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Res 1029:135–139
Konorski J (1967) Integrative activity of the brain; an interdisciplinary approach. University of Chicago Press, Chicago
Krause M, German PW, Taha SA, Fields HL (2010) A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci 30:4746–4756
Krebs H, Macht M, Weyers P, Weijers HG, Janke W (1996) Effects of stressful noise on eating and non-eating behavior in rats. Appetite 26:193–202
Krebs H, Weyers P, Macht M, Weijers HG, Janke W (1997) Scanning behavior of rats during eating under stressful noise. Physiol Behav 62:151–154
Krook-Magnuson E, Luu L, Lee SH, Varga C, Soltesz I (2011) Ivy and neurogliaform interneurons are a major target of mu-opioid receptor modulation. J Neurosci 31:14861–14870
Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ (2014) Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci 17:248–253
Leibowitz SF, Hor L (1982) Endorphinergic and alpha-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides 3:421–428
Levine AS, Murray SS, Kneip J, Grace M, Morley JE (1982) Flavor enhances the antidipsogenic effect of naloxone. Physiol Behav 28:23–25
Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ (1995) Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Phys 268:R248–R252
Love TM, Stohler CS, Zubieta JK (2009) Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry 66:1124–1134
Lynch WC (1986) Opiate blockade inhibits saccharin intake and blocks normal preference acquisition. Pharmacol Biochem Behav 24:833–836
Mahler SV, Berridge KC (2012) What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology 221:407–426
Majeed NH, Przewlocka B, Wedzony K, Przewlocki R (1986) Stimulation of food intake following opioid microinjection into the nucleus accumbens septi in rats. Peptides 7:711–716
Maldonado-Irizarry CS, Swanson CJ, Kelley AE (1995) Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15:6779–6788
McElroy SL, Guerdjikova AI, Blom TJ, Crow SJ, Memisoglu A, Silverman BL, Ehrich EW (2013) A placebo-controlled pilot study of the novel opioid receptor antagonist ALKS-33 in binge eating disorder. Int J Eat Disord 46:239–245
McQuiston AR, Saggau P (2003) Mu-opioid receptors facilitate the propagation of excitatory activity in rat hippocampal area CA1 by disinhibition of all anatomical layers. J Neurophysiol 90:1936–1948
Mena JD, Sadeghian K, Baldo BA (2011) Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci 31:3249–3260
Mena JD, Selleck RA, Baldo BA (2013) Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci 33:18540–18552
Mitchell JM, Tavares VC, Fields HL, D’Esposito M, Boettiger CA (2007) Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology 32:439–449
Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL (2012) Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med 4:116ra6
Mogenson GJ, Swanson LW, Wu M (1983) Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci 3:189–202
Morganstern I, Liang S, Ye Z, Karatayev O, Leibowitz SF (2012) Disturbances in behavior and cortical enkephalin gene expression during the anticipation of ethanol in rats characterized as high drinkers. Alcohol 46:559–568
Mucha RF, Iversen SD (1986) Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res 397:214–224
Nowend KL, Arizzi M, Carlson BB, Salamone JD (2001) D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav 69:373–382
O’Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, Luscher C (2015) Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88:553–564
Onuki Y, Makino J (2005) Food-carrying behavior increased under risk-approaching signal in rats (Rattus norvegicus). Physiol Behav 84:141–145
Ostlund SB, Kosheleff A, Maidment NT, Murphy NP (2013) Decreased consumption of sweet fluids in mu opioid receptor knockout mice: a microstructural analysis of licking behavior. Psychopharmacology 229:105–113
Parent MA, Amarante LM, Liu B, Weikum D, Laubach M (2015) The medial prefrontal cortex is crucial for the maintenance of persistent licking and the expression of incentive contrast. Front Integr Neurosci 9:23
Parker LA, Maier S, Rennie M, Crebolder J (1992) Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behav Neurosci 106:999–1010
Parker KE, McCabe MP, Johns HW, Lund DK, Odu F, Sharma R, Thakkar MM, Cornelison DD, Will MJ (2015) Neural activation patterns underlying basolateral amygdala influence on intra-accumbens opioid-driven consummatory versus appetitive high-fat feeding behaviors in the rat. Behav Neurosci 129:812–821
Pecina S, Berridge KC (1995) Central enhancement of taste pleasure by intraventricular morphine. Neurobiology (Bp) 3:269–280
Pecina S, Berridge KC (2005) Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci 25:11777–11786
Pecina S, Berridge KC (2013) Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci 37:1529–1540
Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053
Petrovich GD, Holland PC, Gallagher M (2005) Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci 25:8295–8302
Petrovich GD, Ross CA, Holland PC, Gallagher M (2007) Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci 27:6436–6441
Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA (2006) The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol 26:610–625
Picetti R, Schlussman SD, Zhou Y, Ray B, Ducat E, Yuferov V, Kreek MJ (2013) Addictions and stress: clues for cocaine pharmacotherapies. Curr Pharm Des 19:7065–7080
Plaza-Zabala A, Maldonado R, Berrendero F (2012) The hypocretin/orexin system: implications for drug reward and relapse. Mol Neurobiol 45:424–439
Quirk GJ, Garcia R, Gonzalez-Lima F (2006) Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60:337–343
Ragozzino ME, Detrick S, Kesner RP (1999) Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci 19:4585–4594
Reppucci CJ, Petrovich GD (2016) Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct 221:2937–2962
Rhodes SE, Killcross AS (2007) Lesions of rat infralimbic cortex enhance renewal of extinguished appetitive Pavlovian responding. Eur J Neurosci 25:2498–2503
Richard JM, Berridge KC (2013) Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biol Psychiatry 73:360–370
Rideout HJ, Parker LA (1996) Morphine enhancement of sucrose palatability: analysis by the taste reactivity test. Pharmacol Biochem Behav 53:731–734
Robinson TE, Berridge KC (2001) Incentive-sensitization and addiction. Addiction 96:103–114
Rudski JM, Billington CJ, Levine AS (1994) Naloxone’s effects on operant responding depend upon level of deprivation. Pharmacol Biochem Behav 49:377–383
Salamone JD, Cousins MS, Bucher S (1994) Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res 65:221–229
Salamone JD, Correa M, Farrar A, Mingote SM (2007) Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology 191:461–482
Schienle A, Schafer A, Hermann A, Vaitl D (2009) Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry 65:654–661
Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ (2008) The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett 432:40–45
Selleck RA, Lake C, Estrada V, Riederer J, Andrzejewski M, Sadeghian K, Baldo BA (2015) Endogenous opioid signaling in the medial prefrontal cortex is required for the expression of hunger-induced impulsive action. Neuropsychopharmacology 40:2464–2474
Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R (2013) Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry 70:727–739
Soyka M, Rosner S (2008) Opioid antagonists for pharmacological treatment of alcohol dependence—a critical review. Curr Drug Abuse Rev 1:280–291
Spector AC, Klumpp PA, Kaplan JM (1998) Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci 112:678–694
Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF (1993) The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s). Brain Res 604:304–317
Stratford TR, Kelley AE (1999) Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci 19:11040–11048
Stratford TR, Swanson CJ, Kelley A (1998) Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav Brain Res 93:43–50
Taki K, Kaneko T, Mizuno N (2000) A group of cortical interneurons expressing mu-opioid receptor-like immunoreactivity: a double immunofluorescence study in the rat cerebral cortex. Neuroscience 98:221–231
Thompson RH, Swanson LW (2010) Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc Natl Acad Sci U S A 107:15235–15239
Toates FM (1986) Motivational systems. Cambridge University Press, Cambridge Cambridgeshire; New York
Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, Andrew CM, Williams SC, Campbell IC, Treasure J (2004) Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry 161:1238–1246
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58
Volpicelli JR (1995) Naltrexone in alcohol dependence. Lancet 346:456
Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP (1992) Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 49:876–880
Weldon DT, O’Hare E, Cleary J, Billington CJ, Levine AS (1996) Effect of naloxone on intake of cornstarch, sucrose, and polycose diets in restricted and nonrestricted rats. Am J Phys 270:R1183–R1188
Will MJ, Franzblau EB, Kelley AE (2003) Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci 23:2882–2888
Will MJ, Franzblau EB, Kelley AE (2004) The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport 15:1857–1860
Wilson JD, Nicklous DM, Aloyo VJ, Simansky KJ (2003) An orexigenic role for mu-opioid receptors in the lateral parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 285:R1055–R1065
Woods JS, Leibowitz SF (1985) Hypothalamic sites sensitive to morphine and naloxone: effects on feeding behavior. Pharmacol Biochem Behav 23:431–438
Woolley JD, Lee BS, Fields HL (2006) Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience 143:309–317
Yeomans MR, Gray RW (1996) Selective effects of naltrexone on food pleasantness and intake. Physiol Behav 60:439–446
Zhang M, Kelley AE (2000) Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience 99:267–277
Zhang M, Kelley AE (2002) Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology 159:415–423
Zhang M, Balmadrid C, Kelley AE (2003) Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci 117:202–211
Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR (2003) Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol 284:R1436–R1444
Zheng H, Patterson LM, Berthoud HR (2007) Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci 27:11075–11082
Zhou L, Sun WL, See RE (2011) Orexin receptor targets for anti-relapse medication development in drug addiction. Pharmaceuticals (Basel) 4:804–821
Ziauddeen H, Chamberlain SR, Nathan PJ, Koch A, Maltby K, Bush M, Tao WX, Napolitano A, Skeggs AL, Brooke AC, Cheke L, Clayton NS, Sadaf Farooqi I, O’Rahilly S, Waterworth D, Song K, Hosking L, Richards DB, Fletcher PC, Bullmore ET (2013) Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry 18:1287–1293
Zieglgansberger W, French ED, Siggins GR, Bloom FE (1979) Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science 205:415–417
Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ (1996) Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med 2:1225–1229
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selleck, R.A., Baldo, B.A. Feeding-modulatory effects of mu-opioids in the medial prefrontal cortex: a review of recent findings and comparison to opioid actions in the nucleus accumbens. Psychopharmacology 234, 1439–1449 (2017). https://doi.org/10.1007/s00213-016-4522-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4522-4