Abstract
This study explored potentially dissociable functions of mu-opioid receptor (µ-OR) signaling across different cortical territories in the control of anticipatory activity directed toward palatable food, consumption, and impulsive food-seeking behavior in male rats. The µ-OR agonist, DAMGO ([D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin), was infused into infralimbic cortex (ILC), prelimbic cortex (PrL), medial and lateral ventral orbitofrontal cortices (VMO, VLO), and agranular/dysgranular insular (AI/DI) cortex of rats. Intra-ILC DAMGO markedly enhanced contact with a see-through screen behind which sucrose pellets were sequestered; in addition, rats having received intra-ILC and intra-VMO DAMGO exhibited locomotor hyperactivity while the screen was in place. Upon screen removal, intra-ILC and -VMO-treated rats emitted numerous, brief sucrose-intake bouts (yielding increased overall intake) interspersed with significant hyperactivity. In contrast, intra-AI/DI-treated rats consumed large amounts of sucrose in long, uninterrupted bouts with no anticipatory hyperactivity pre-screen removal. Intra-PrL and intra-VLO DAMGO altered neither pre-screen behavior nor sucrose intake. Finally, all rats were tested in a sucrose-reinforced differential reinforcement of low rates (DRL) task, which assesses the ability to advantageously withhold premature responses. DAMGO affected (impaired) DRL performance when infused into ILC only. These site-based dissociations reveal differential control of µ-OR-modulated appetitive/approach vs. consummatory behaviors by ventromedial/orbitofrontal and insular networks, respectively, and suggest a unique role of ILC µ-ORs in modulating inhibitory control.
Similar content being viewed by others
Introduction
The motivation to seek and consume food is essential for survival. However, when food motivation is unchecked by normal inhibitory controls, goal-seeking responses become excessive or intrusive, and consumption surpasses homeostatic requirements. A better understanding of the anatomical networks and neurochemical systems that initiate and adaptively limit natural appetitive behaviors, such as feeding, may uncover principles that apply to a wide variety of addictions, eating disorders (EDs), and other conditions with common underlying features of motivational dysfunction.
Decades of research into the central control of feeding behavior has established that mu-opioid receptor (μ-OR) signaling in discrete subcortical loci (including the nucleus accumbens [1], central amygdala [2], and hypothalamus [3]) is sufficient to drive intense feeding responses, including for palatable or preferred foods [4,5,6]. The feeding-modulatory role of cortically localized μ-ORs, however, has been comparatively understudied. Nevertheless, recent work has begun to identify cortical loci, in which μ-OR stimulation produces motivational effects rivaling those seen in extensively studied subcortical sites, such as the nucleus accumbens. For example, μ-OR stimulation in a circumscribed area of ventromedial prefrontal cortex (vmPFC), alone among a variety of monoamine or amino acid neurotransmitter manipulations, augments the intake of both standard and palatable food, selectively enhances feeding in a concurrent food/water choice in fluid-restricted rats, and robustly increases the willingness to work for sucrose reward in a progressive-ratio task, even in rats with low hunger drive [7,8,9]. Furthermore, intra-vmPFC μ-OR signaling, but not monoamine stimulation, produces a marked impulsivity-like impairment of inhibitory control in a sucrose-reinforced differential reinforcement of low rates (DRL) task, which assesses the ability to withhold disadvantageous, prepotent food-seeking responses [9]. To date, no other neurochemical manipulation of the vmPFC has been found to produce this behavioral profile. Hence, μ-OR stimulation may represent a unique tool with which to “push” cortical sites to reveal feeding-related functions. Accordingly, recent studies have begun to identify additional loci beyond vmPFC, in rat ventral orbitofrontal and posterior insular cortex, where μ-OR stimulation enhances feeding and related functions, such as hedonic taste reactivity [10]. The clinical relevance of these observations is bolstered by the growing number of human ligand-PET and fMRI neuroimaging studies showing μ-OR changes within homologous medial, orbitofrontal, and insular cortical regions in conditions pertinent to appetitive motivation and its dysregulation, such as feeding and viewing food images [11, 12], obesity and EDs [13,14,15], various stages of the drug addiction cycle [16,17,18,19], and impulsivity [20].
To better understand the functions of cortical μ-OR systems, the present study mapped frontal-cortical μ-OR agonist actions on dissociable components of palatable food-motivated behavior: behavior antecedent to expected food access, actual consumption, and inhibitory control over food-seeking behavior. Our overarching goal was to determine the extent to which these functions are either overlapping or segregated to distinct nodes in frontal-cortical circuitry. Small-volume infusions of the selective μ-OR agonist, DAMGO ([D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin), were placed in rat vmPFC or dorsomedial prefrontal cortex (infralimbic cortex (ILC) and prelimbic cortex (PrL) regions, respectively), orbitofrontal cortex (ventromedial (VMO) and lateral orbitofrontal regions (VLO)), or insular cortex (agranular/dysgranular regions at the level of primary gustatory cortex (AI/DI)). All these sites have appreciable densities of μ-ORs, with no evidence for major differences in receptor expression across sites [21, 22]. Using a within-subjects design, rats were tested in two paradigms: an observational test in which spontaneous behaviors prior to expected sucrose access and during access were recorded and scored; and the abovementioned DRL task of inhibitory control. Of particular interest was determining the degree to which cortical sites subserving μ-OR-mediated effects on inhibitory control anatomically overlap the sites that generate anticipatory activity, consumption, or both.
Methods
Subjects
Subjects were male Sprague–Dawley rats (Harlan, Madison, WI) weighing 275–300 g upon arrival at the laboratory. Rats were housed in a light- and temperature-controlled vivarium, under a 12:12 h light–dark cycle (lights on at 0700 h). Food (Envigo Teklad laboratory diet) and water were available ad libitum, except as indicated for specific experiments. Animals were handled daily to reduce stress. Testing occurred between 1100 and 1500 h. All facilities and procedures were in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committee of Univ. Wisconsin-Madison.
Differential reinforcement of low response rates (DRL-15 s)
Training and testing procedures were conducted in standard operant chambers, as described previously [9].
All rats underwent an initial training period during which they were maintained at 90 ± 2% of free-feeding body weight using scheduled feedings. During this phase, rats were trained to lever press on a conjoint random-time 30 s/fixed ratio 1 schedule (RT-30 s/FR-1) and then a FR-1 schedule [9] to earn sucrose pellets (BioServe Dustless Precision Sucrose Pellets, 45 mg; Product # F0023). Once consistent responding was achieved on FR-1, rats advanced to a variable-interval 15 s schedule (VI-15 s), VI-30 s schedule, and finally to a DRL-15 s schedule. In the DRL-15 s schedule, rats were required to withhold responding after reinforcer delivery during an unsignaled, fixed time period (15 s). After this delay, subjects could respond to earn the next reinforcer. However, each premature response (i.e., one that was not separated from the previous response by at least 15 s) reset the delay timer. For optimal performance, therefore, the timing of consecutive responses (inter-response intervals) must exceed the delay interval. After 2–3 days on DRL-15 s, rats were returned to ad libitum feeding, with food withdrawn in the 2 h immediately preceding each testing session. Rats were then tested under this new food restriction schedule until stable baseline responding was achieved (±15% variability or less) in reinforced responses over three consecutive testing days. DRL sessions lasted ~20 min.
Behavior-observation procedure
Rats were tested in clear polycarbonate cages (9.5 in width × 17 in length × 8 in height), identical to their home cages except for wire grid floors. Pre-measured amounts of sucrose pellets (the same type of sucrose pellets that were used in the DRL procedure described above) were placed in clear glass jars affixed to the wire grid. For the first part of the session, chicken-wire screens blocked access to the sucrose, while allowing it to be seen. All sessions started with screens in place; screens were removed after 15 min to allow 45 min of access to and consumption of the sucrose pellets. Rats were videotaped with a digital camcorder for the entire 60 min session (i.e., 15 min with screens in place, 45 min after screen removal). After the conclusion of testing, an experimenter blind to treatment viewed the digital files, and recorded number and length of bouts of screen contacts, spontaneous ambulation (cage crossings), rearing, and eating, using an event recorder interfaced to a PC-based desktop computer. Several derivative measures were calculated from these data: mean eating bout duration (total # of bouts/total duration), global eating rate (total intake/total duration), and global eating efficiency (total intake/total # of bouts). To record the duration of a particular behavioral event, a cumulative timer (specific for that behavior) on the event recorder was started at the initiation of the behavior. The timer was switched off when the behavior was interrupted by a different behavior.
Surgical procedures
Stereotaxic surgery under isoflurane anesthesia was carried out, as described previously [23]. Stainless-steel guide cannulae (10-mm-long; 25-gauge) were implanted at flat skull aiming at ILC (N = 7 rats; AP +3.0, ML ±2.25, DV −5.2, at 19° from vertical from skull surface), PrL (N = 8 rats; AP +3.0, ML ±2.0, DV −5.15, at 23° from vertical, from skull surface), VMO cortex (N = 9 rats; AP +3.25, ML ±1.7, DV −5.15, from skull surface), VLO cortex (N = 7 rats; AP +3.2, ML ±2.8, DV −5.15, from skull surface), and AI/DI cortex (N = 7 rats; AP +1.3, ML ±5.05, DV –6.65, from skull surface). All guide cannulae were implanted 2.5 mm above the final infusion site except for PrL, which is very dorsal, where injectors extended 1 mm beyond the guide cannulae.
Microinfusion procedures and drugs
Intracerebral microinjections were carried out, as previously described [23]. DAMGO was obtained from Bachem. All drugs were dissolved in sterile 0.9% saline.
Experimental design
After a week of recovery from surgery, rats were separated into two groups tested either first in DRL-15 s followed by the behavior-observation procedure, or vice versa, in a counterbalanced block design. All rats were first acclimated to microinjection procedures with sham infusions and intra-site saline injections on consecutive days. Drug testing in either DRL or the behavior-observation procedure commenced 2 days after acclimation. Rats received intracranial infusions of either vehicle (0.9% saline) or DAMGO (0.1, 0.3, or 1.0 μg). The microinfusion volume was 0.25 μl/side. Each rat received all those treatments counterbalanced according to latin square designs within each block. Testing began 15 min after microinfusions. At least 1 day was allowed between treatments to allow for drug washout. At the conclusion of dose-response testing, the blocks were reversed, and dose-response testing commenced again.
Histology
At the end of each experiment, rats were deeply anesthetized and perfused with 10% formalin in phosphate buffer. Brains were stored in 10% formalin. Coronal sections (60 µm) were cut through the infusion site on a cryostat microtome, collected on slides, stained with cresyl violet, and subsequently reviewed to verify correct injection placements.
Statistical analyses
Data were analyzed using repeated measures ANOVAs. Contingent upon significance in the ANOVAs, post hoc comparisons among means were conducted using Tukey’s HSD test. The level of statistical significance was set at P < 0.05 for all experiments.
Results
Histological analyses
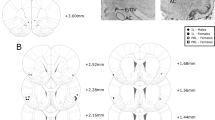
Chartings in Fig. 1A–E depict the placement of injector tips in each of the targeted cortical sites for rats included in the study. Bilateral placements within each rat were never separated from one another by >0.4 mm in the anteroposterior plane. The photomicrographs in Fig. 1F shows a representative high-power view of injector tip placements in Nissl (cresyl violet)-stained tissue. For included rats, no atypical or excessive damage was noted; examination of the area even closely adjacent to the injector tips revealed intact cells and good tissue integrity (see brightfield photomicrographs in Fig. 1F).
A ILC infralimbic cortex (N = 7). B PrL prelimbic cortex (N = 8). C VMO ventromedial orbitofrontal cortex (N = 9). D VLO ventrolateral orbitofrontal cortex (N = 7). E AI/DI agranular/dysgranular insular cortex (N = 7). Numbers indicate distance from bregma in millimeters. F High-power view of an injector tip, showing no unusual damage and good cell integrity at the injection site.
Order of participation in DRL vs. the behavioral-observation procedure had no effect
All rats were subjected to a within-subject counterbalanced design, in which they were tested in either a behavioral-observation testing block first, and operant DRL-15 s testing block second, or vice versa (see “Methods”). No effects of block order were detected for any site or measure (Fs = 0.06–2.54, not significant (NS)).
Intra-ILC and intra-VMO DAMGO-modulated behavior prior to contact with food
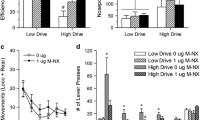
Dissociable effects of DAMGO were noted across cortical sites in the first 15 min of the behavioral-observation test, while the wire-mesh screen preventing food contact was still in place. Intra-ILC DAMGO increased the number of screen contacts (F(3,18) = 3.22, P = 0.047; Fig. 2A), which consisted of sniffing and contacting the screen with the paws (i.e., treating the screen as a barrier), but with no licking, gnawing, or other obvious ingestive-behavior fragments. No statistically significant effects of DAMGO on screen contacts were detected for any of the other sites (Fs = 0.06–2.30, NS). In addition, DAMGO increased the number of cage crossings while the screen was in place for ILC (F(3,18) = 8.05, P = 0.001) and VMO (F(3,24) = 5.58, P = 0.005), but not for PrL, VLO, or AI/DI (Fs = 0.26–2.11; NS, Fig. 2B).
A Screen contacts and B locomotor activity are shown. * Different than saline (P < 0.05), ** different than saline (P < 0.01), ## different than 0.1 µg DAMGO (P < 0.01). Error bars depict 1 SEM. ILC infralimbic cortex, PrL prelimbic cortex, VMO ventromedial orbitofrontal cortex, VLO ventrolateral orbitofrontal cortex, AI/DI agranular/dysgranular insular cortex.
Intra-site DAMGO infusions augmented sucrose-pellet intake in ILC, VMO, and AI/DI
In the 45-min period after the screen was removed from the sugar pellets, DAMGO microinfusions significantly increased sucrose intake in three of the five sites tested (see Fig. 3). For sub-territories of medial PFC, DAMGO infusions into ILC elicited significant sucrose hyperphagia (F(3,18) = 8.31, P = 0.001), while no effects were noted more dorsally in PrL (F(3,21) = 0.38, NS). In orbitofrontal cortex, a DAMGO-induced increase in sucrose intake was observed in VMO (F(3,24) = 3.03, P = 0.049), but not more laterally in VLO (F(3,18) = 1.79, NS). The magnitude of DAMGO’s effect on intake was similar in ILC and VMO, averaging 4.8 and 4.3 g of sucrose, respectively, after the 1.0 µg dose. Intra-AI/DI DAMGO infusions also elicited considerable sucrose hyperphagia (F(3,18) = 9.08, P = 0.0007), with intake at the 1.0 µg dose averaging 7.1 g.
**P < 0.01, ***P < 0.001, different from saline. #P < 0.05, ##P < 0.01, ###P < 0.001 different than 0.1 µg DAMGO. Error bars depict 1 SEM. ILC infralimbic cortex, PrL prelimbic cortex, VMO ventromedial orbitofrontal cortex, VLO ventrolateral orbitofrontal cortex, AI/DI agranular/dysgranular insular cortex.
DAMGO provoked dissociable feeding-behavior patterns across cortical sites
In the 45-min post-screen testing period, intra-ILC DAMGO-induced augmentation of sucrose intake was characterized by a significantly higher number of eating bouts initiated (F(3,18) = 3.65, P = 0.032; Fig. 4A), a significant but small increase in total eating duration (F(3,18) = 3.77, P = 0.029; Fig. 4B). These changes resulted in a significant reduction in mean eating bout duration (F(3,18) = 7.14, P = 0.0023), and corresponding reduction in gram intake per bout (F(3,18) = 5.20, P = 0.009); however, global eating rate (gram intake/min) was unchanged. These findings are summarized in Supplementary Materials, Table S1. Also shown in Fig. 4B, DAMGO increased the number of eating bouts when infused into VMO (F(3,24) = 6.83, P = 0.002) or VLO (F(3,18) = 3.79, P = 0.029); however, effects in these sites on eating duration trended upward slightly but did not achieve statistical significance (Fs = 0.91–2.38, NS; Fig. 4B). Similarly to ILC, mean eating bout duration was significantly decreased in VMO (F(3,24) = 8.09, P = 0.0007; Supplementary Materials, Table S1). Moreover, global eating rate (gram intake per minute) was significantly increased in VMO alone among all sites (F(3,24) = 3.44, P = 0.033; Supplementary Materials, Table S1). Together, these results indicate that feeding responses elicited from ILC and VMO were intense, but very brief and fragmentary. The opposite pattern was seen in AI/DI, where DAMGO had a relatively small but still significant effect on number of eating bouts (F(3,18) = 3.38, P = 0.041), while more than doubling total eating duration (F(3,18) = 8.56, P = 0.001; Fig. 4A, B). Mean eating bout duration, gram intake per bout, and global eating rate also were dose-relatedly increased in AI/DI, although these effects were not statistically significant (Fs = 1.58–1.67, NS; Supplementary Materials, Table S1). Finally, in PrL, DAMGO failed to alter eating bouts, duration, global eating rate or efficiency (Fs = 0.17–1.35, NS), but produced a small but significant decrease in mean bout duration at the highest dose (F(3,21) = 3.81, P = 0.025); these findings are shown in Fig. 4 and in Supplementary Materials, Table S1.
Main figures in A and B depict eating bouts and eating duration, respectively, for the entire 45-min post-screen testing sessions, while Insets depict the first 10 min of the sessions. *, ** Different than saline P < 0.05 and P < 0.01, ## different than 0.1 µg DAMGO P < 0.01, ‡‡‡ different than all other treatments P ≤ 0.001. Error bars depict 1 SEM. ILC infralimbic cortex, PrL prelimbic cortex, VMO ventromedial orbitofrontal cortex, VLO ventrolateral orbitofrontal cortex, AI/DI agranular/dysgranular insular cortex.
Overall, the same behavioral patterns and site dissociations described above for the entire 45-min post-screen session had begun to appear as early as the first 10 min of the 45-min post-screen periods (see Fig. 4 insets). Thus, DAMGO increased the number of eating bouts when infused into ILC (F(3,18) = 4.65, P = 0.001) and VMO (F(3,24) = 8.83, P = 0.0004). These effects on bout initiation did not translate into statistically significant increases in overall eating duration (Fs = 0.47–1.23, NS), indicating that the feeding bouts were very short and fragmentary. This conclusion was further bolstered by the finding that mean bout duration in the ILC was significantly decreased (F(3,18) = 5.13, P = 0.0097; Supplementary Materials, Table S1). In contrast, DAMGO infusions into AI/DI did not increase the number of eating bouts initiated in the first 10 min of the session (F(3,18) = 0.67, NS), but greatly increased feeding duration (F(3,18) = 5.99, P = 0.005). These analyses confirm the qualitative observation that, after screen removal, intra-AIC DAMGO-treated rats moved directly into the sugar jars and consumed the pellets in long, uninterrupted bouts, in strong contrast to the “fragmented” behavioral pattern observed after DAMGO infusions into ILC or VMO.
DAMGO microinfusions elicited motor hyperactivity in the presence of food
In the 45-min post-screen period, DAMGO elicited hyperactivity, indexed by cage crossings, in ILC (F(3,18) = 8.01, P = 0.001), VMO (F(3,24) = 5.90, P = 0.004), VLO (F(3,18) = 7.36, P = 0.002), and PrL (F(3, 21) = 3.74, P = 0.003); all shown in Supplementary Materials, Fig. S1. In contrast, infusions of DAMGO into the AI did not alter total cage crossings, (F(3,18) = 1.26, NS) despite strong modulation of feeding at the same doses. To adjust for the different overall feeding durations obtained in different sites, we calculated the number of cage crossings per unit time the animals were not eating (i.e., cage crossings/[total session duration − total eating duration]), which provided an index of locomotion rate. This adjustment revealed significant dose-related increases in locomotion rates across sites (ILC: F(3,18) = 6.39, P = 0.0039; VMO: F(3,24) = 4.94, P = 0.0078; VLO: F(3,18) = 7.72, P = 0.0016, PrL: F(3,21) = 3.98, P = 0.022, AI/DI: F(3,18) = 6.03, P = 0.005). The rank order of highest mean locomotion rate across sites (crossings/min) was ILC (3.36) > VMO (2.52) > VLO (1.71) > AI/DI (1.29) > PrL (1.09). Hence, the largest increases in locomotion (both total locomotion and locomotion rate during nonfeeding time) were seen in ILC and VMO. These results are shown in Supplementary Materials, Table S1.
Except in PrL and AI (Fs = 0.28–1.30, NS), DAMGO significantly increased general activity in the first 10 min of the 45-min post-screen sessions (Fs = 3.28–6.55, Ps = 0.004–0.038), as shown in the Supplementary Materials, Fig. S1.
Intra-site DAMGO impaired DRL-15 performance in ILC only
Performance in the DRL-15 task was indexed by analyzing premature responses (disadvantageous responses emitted during the 15-s waiting period), nose pokes into the sucrose-pellet hopper (an index of generalized motivational and motoric activation), and response efficiency (the proportion of total responses that were successfully reinforced (shown in Fig. 5)). As responding becomes impulsive-like, premature responding increases and efficiency correspondingly decreases. DAMGO dose-relatedly decreased efficiency and increased premature responding only when infused into ILC (F(6,18) = 5.57, P = 0.007 for response efficiency; F(6,18) = 3.92, P = 0.026 for premature responses). No DAMGO-induced effects on either of those two measures were noted in any other site (Fs = 0.10–2.84, NS). Furthermore, there were no significant effects on nose pokes in any site (Fs = 0.81–2.60, NS). Premature responses and nose pokes are summarized in Supplementary Materials, Table S2.
An increase in disadvantageous, premature responding results in a reduction of response efficiency (see “Methods” for further details). **P < 0.01. Error bars depict 1 SEM. ILC infralimbic cortex, PrL prelimbic cortex, VMO ventromedial orbitofrontal cortex, VLO ventrolateral orbitofrontal cortex, AI/DI agranular/dysgranular insular cortex.
Discussion
The present study revealed marked heterogeneity in the behavioral profiles elicited by µ-OR stimulation across the cortical sites tested. Infusions of DAMGO into ILC and VMO, but not VLO, PrL, or AI/DI, provoked considerable hyperactivity prior to feeding, when a sucrose-filled jar was covered by a see-through mesh screen (and rats had previously learned to expect eventual screen removal). Intra-ILC DAMGO also increased the number of times rats contacted the screen. After screen removal, intra-ILC, intra-VMO, and intra-AI/DI DAMGO-treated rats exhibited sucrose hyperphagia; however, microanalysis of feeding behavior revealed markedly dissimilar behavioral patterns across those sites. Whereas intra-ILC and intra-VMO DAMGO infusions produced numerous, brief, feeding bouts interspersed with frequent bouts of intense hyperactivity, intra-AI/DI DAMGO elicited long, uninterrupted feeding bouts with no hyperactivity before screen removal. In contrast, intra-VLO and intra-PrL DAMGO provoked a modest increase in locomotor activity (only in the presence of food), but no changes in feeding. Finally, DAMGO infusion into ILC but no other site impaired DRL responding by provoking premature-response errors. These results reveal functional specializations across frontal territories in µ-OR modulation of activity and approach, intake, and inhibitory control over food-seeking responses.
Prior intra-cortical DAMGO infusion studies support the present findings of DAMGO-responsive zones in medial prefrontal, orbital, and insular cortices, albeit with some interesting differences. A more limited mapping from our prior work showed sites of DAMGO-driven food intake clustered around the ILC/PrL boundary and VMO, but not control sites in motor cortex [7]. A more recent study reported that DAMGO augmented both intake and hedonic taste reactivity in medial and lateral orbitofrontal cortex; many of these placements were in the general vicinity of the VMO site probed here, but slightly more anterior [10]. These investigators also report some strong DAMGO-induced feeding responses clustered in the medial wall of PFC, ~0.5–1.0 mm anterior to the current ILC site (see Fig. 7 in ref. [10]). However, it is unclear whether these investigators probed the specific ILC site tested here. Finally, in insular cortex, sharp anteroposterior gradients of DAMGO-induced intake, hedonic enhancement, and hedonic suppression were found, in which placements ~2.0–3.0 mm posterior to the site probed here strongly augmented hedonic taste reactivity while producing little effect on intake, while more anterior placements (including the level probed here, where we obtained pronounced hyperphagia) yielded some inconsistent increases in food intake and, interestingly, hedonic suppression. Together, these findings converge on a ventrally localized DAMGO-responsive corridor comprising medial sectors of orbitofrontal and anterior sectors of vmPFC, with gradients of increasing sensitivity as placements move rostrally and ventrally in medial prefrontal cortex, and rostrally and medially in orbitofrontal cortex. Regarding insular cortex, there is evidence that µ-OR stimulation amplifies food intake; however, more work is needed to resolve the anatomical boundaries of this effect more precisely.
Among the most striking of the behavioral dissociations observed in the present study was the difference in the patterns elicited from ILC and VMO vs. AI/DI. Overall, the present results suggest that µ-OR signaling in ILC and VMO (but less so in VLO, and not at all in PrL) is sufficient to generate significant hyperactivity and a food-seeking motivational state, in general agreement with prior findings that intra-IL DAMGO amplifies effortful sucrose-seeking operant behavior [8, 9] and that medial PFC is strongly activated by food-directed exploratory activity and less so (although still significantly) during consummatory repertoires [24]. Furthermore, the present data bolster prior observations that the overall behavioral phenotype observed after DAMGO stimulation of ventromedial prefrontal and medial orbitofrontal territories is disorganized and fragmented, with bouts of exploratory-like behaviors interrupting bouts of sucrose intake [7]. Nevertheless, despite the similar “fragmented” behavioral patterns elicited from ILC and VMO, impairment of DRL responding was observed only after ILC infusions. In fact, ILC was the only site where DAMGO infusions altered DRL performance; infusions just 1.0 mm dorsal (PrL) or 1.0 mm medial (VMO) did not significantly alter responding, highlighting the circumscribed nature of the effect. Combining results from all the current activity/feeding and DRL experiments reveals the functional specificity of the DRL impairment; neither DAMGO-induced sucrose hyperphagia, nor the generation of hyperactivity either in anticipation of or during sucrose access, represented conditions sufficient to alter DRL responding. Hence, the ILC-localized DRL impairment was not an artifact of generalized arousal or motor activation, nor the obligatory outcome of enhanced feeding motivation. Conversely, when ILC was unperturbed, DRL response efficiency was preserved even in the face of the amplified food motivation and/or hyperactivity variously elicited from the other sites. Studies employing the five-choice serial reaction time task (5-CSRTT) have yielded closely comparable results (like the DRL deficits shown here, premature responding in the 5-CSRTT is considered an index of “waiting” or “action” impulsivity [25]). Lesions of ILC but not of other frontal sites, or glutamatergic or GABA-ergic manipulations of the IL (none of which overtly enhance appetitive motivation), engender marked premature responding in the 5-CSRTT [26, 27]. More generally, it has been hypothesized that ILC contributes to response selection by restraining prepotent or contextually disadvantageous learned responses, including in extinction [25, 28,29,30,31]. Prior neuropharmacological studies of this inhibitory-control function have focused on dopamine, serotonin, and acetylcholine systems [25]. The present results additionally suggest that supernormal µ-OR signaling in the ILC also plays a role, while simultaneously engaging a motivational function (general motivational arousal, “seeking-like” behavior, and disorganized feeding responses) that is more widely distributed through vmPFC plus parts of orbitofrontal cortex, but which is not in itself sufficient to impair inhibitory control in the DRL task.
In contrast to ILC and VMO, µ-OR stimulation in AI/DI affected behavior only during sucrose access, and the ingestive pattern consisted of long, uninterrupted bouts of intake. Considering that the level of insular cortex targeted here corresponds to primary gustatory cortex [32,33,34,35], one explanation for this behavioral pattern is that DAMGO enhanced the sensory perception and/or the hedonic evaluation of sucrose actively being tasted. Enhancement of either sensory processing or palatability perception could prolong bouts of contact and ingestion, which is one effect of increasing the concentration and presumably the reward value of sugar [36]. Accordingly, electrophysiological recording studies have identified units and ensembles at a level of insular cortex corresponding to the present infusions that encode chemosensory tasteant characteristics and/or palatability [37,38,39,40]. Relatedly, prior work from our group showed that GABA-mediated inactivation of this general area significantly decreased intake of sweet liquid food but not of standard chow pellets [41]. The contrasting behavioral functions of AI/DI-localized µ-ORs and GABA receptors (i.e., hyper- and hypophagia, respectively) could be due to the fact that µ-ORs negatively modulate cortical inhibitory interneurons, thereby disinhibiting local network function [42,43,44,45]) in a manner similar to well-established µ-OR actions in hippocampus [46,47,48]. In contrast, intra-cortical GABA receptor stimulation causes global inhibition of local cortical cellular networks [49, 50].
Units in gustatory cortex also respond to stimuli antecedent to feeding, including food-predictive Pavlovian cues [51], and there is considerable evidence from human neuroimaging studies that insular cortex responds to food images and other food-associated second-order stimuli [52,53,54]. In this context, the absence of intra-AI/DI DAMGO effects on hyperactivity during anticipation of feeding is noteworthy, particularly given the extensive one- and two-stage connections with the orbitofrontal and medial prefrontal areas that generated anticipatory activity in the present study [33]. The mechanism underlying this behavioral specificity in AI/DI is unclear, but it implies a selective action of µ-ORs on local ensemble dynamics specific to ongoing consummatory activity, specialized µ-OR modulation of output pathways mediating consummatory action patterns, or a combination of the two. µ-OR signaling could also modify a “supervisory” function, such as switching out of eating bouts once they have begun. These effects could modify the contribution of AI/DI to response-selection networks, with the net effect of focusing overall behavioral output on consumption. Interestingly, recent studies found a causal linkage between a specific pattern of coherent gustatory cortex ensemble activity (interpreted as the emergence of a palatability computation) and the expression of simple orofacial motor patterns to a passively administered tasteant [55, 56], revealing a mechanism in gustatory cortex for top-down sensory control over simple pattern generators. In theory, the putative µ-OR modulation of local circuit dynamics in a way that amplifies palatability computations could enable simple consummatory action patterns to dominate the response repertoire, “short-circuiting” the contributions of competing pathways, and prolonging contact with food, as seen here.
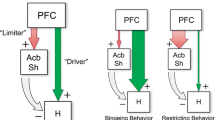
Holistic appetitive-behavior sequences necessitate the integration of dissociable and sometimes competing functions—the active, “seeking-like” repertoires that bring the organism in contact with goal-predictive cues, and, ultimately, with the goal itself; vs. the comparatively simpler consummatory patterns that accompany sensory and reward computations during commerce with the goal. The present results suggest a scheme of frontal-cortical organization in which food approach and “seeking-like” responses are preferentially represented in ventromedial prefrontal and medial orbitofrontal sites, sustained consummatory activity in insular sites, and a dissociable inhibitory-control function in ILC. In a general sense, this segregation of behavioral functions is not incompatible with recent evidence demonstrating µ-OR-driven feeding without taste-reactivity enhancement (“wanting” without “liking”) in medial prefrontal cortex and the converse pattern in posterior insular cortex [10]. It is of great interest to determine how cortical loci studied here interact as a network to merge their respective functions; this could have implications both for understanding how the network generates coherent behavior sequences, as well as how the network learns feeding-related contingencies. For example, prior work has shown that ILC participates in sugar-conditioned flavor preference learning, possibly implicating integration between insular and medial prefrontal networks [57,58,59]. Other important goals include determining the endogenous opioid peptides that act in this network, including those that recruit different intracellular signaling pathways [60,61,62,63]; characterizing the interaction/integration of cortical and subcortical µ-OR actions (for example, between specific cortical sites and the nucleus accumbens); and exploring potential sex differences in the behavioral functions of cortical µ-ORs. Regarding the clinical relevance of the present findings, mounting evidence from human neuroimaging studies indicates that frontal-cortical µ-ORs are involved in EDs and drug addiction [15, 16], and may contribute to an impulsivity endophenotype common to both EDs and addiction [20, 64]. Relatedly, recent studies in rats have demonstrated a strong association between high levels of action impulsivity and binge-like eating patterns [65, 66], and in agreement with the present results, have suggested that projections from the vmPFC to the nucleus accumbens shell regulate both behaviors [66]. The functional heterogeneity observed here may help provide an anatomical framework to further understand these neuroimaging and preclinical results, and to integrate them into a clinical model. For example, using food bingeing as an exemplar, supernormal µ-OR signaling events could selectively (and perhaps sequentially) drive approach toward food (VMO, ILC), degrade the ability to exhibit food restraint once there (ILC), and amplify non-homeostatic feeding once it has begun (AI/DI). Although speculative, this model provides testable predictions regarding the clinical manifestations of localized cortical µ-OR abnormalities.
Funding and disclosure
This work was supported by NIH grant MH074723 from the National Institute for Mental Health. The authors declare no competing interests.
References
Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–77.
Levine AS, Olszewski PK, Mullett MA, Pomonis JD, Grace MK, Kotz CM, et al. Intra-amygdalar injection of DAMGO: effects on c-Fos levels in brain sites associated with feeding behavior. Brain Res. 2004;1015:9–14.
Woods JS, Leibowitz SF. Hypothalamic sites sensitive to morphine and naloxone: effects on feeding behavior. Pharm Biochem Behav. 1985;23:431–8.
Woolley JD, Lee BS, Fields HL. Nucleus accumbens opioids regulate flavor-based preferences in food consumption. Neuroscience. 2006;143:309–17.
Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology. 2002;159:415–23.
Eikemo M, Loseth GE, Johnstone T, Gjerstad J, Willoch F, Leknes S. Sweet taste pleasantness is modulated by morphine and naltrexone. Psychopharmacology. 2016;233:3711–23.
Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by mu-opioid receptor stimulation in circumscribed regions of frontal cortex. J Neurosci. 2011;31:3249–60.
Mena JD, Selleck RA, Baldo BA. Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci. 2013;33:18540–52.
Selleck RA, Lake C, Estrada V, Riederer J, Andrzejewski M, Sadeghian K, et al. Endogenous opioid signaling in the medial prefrontal cortex is required for the expression of hunger-induced impulsive action. Neuropsychopharmacology 2015;40:2464–74.
Castro DC, Berridge KC. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc Natl Acad Sci USA. 2017;114:E9125–E34.
Tuulari JJ, Tuominen L, de Boer FE, Hirvonen J, Helin S, Nuutila P, et al. Feeding releases endogenous opioids in humans. J Neurosci. 2017;37:8284–91.
Nummenmaa L, Saanijoki T, Tuominen L, Hirvonen J, Tuulari JJ, Nuutila P, et al. Mu-opioid receptor system mediates reward processing in humans. Nat Commun. 2018;9:1500.
Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35:3959–65.
Bencherif B, Guarda AS, Colantuoni C, Ravert HT, Dannals RF, Frost JJ. Regional mu-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med. 2005;46:1349–51.
Joutsa J, Karlsson HK, Majuri J, Nuutila P, Helin S, Kaasinen V, et al. Binge eating disorder and morbid obesity are associated with lowered mu-opioid receptor availability in the brain. Psychiatry Res Neuroimaging. 2018;276:41–45.
Kuwabara H, Heishman SJ, Brasic JR, Contoreggi C, Cascella N, Mackowick KM, et al. Mu opioid receptor binding correlates with nicotine dependence and reward in smokers. PLoS ONE. 2014;9:e113694.
Domino EF, Hirasawa-Fujita M, Ni L, Guthrie SK, Zubieta JK. Regional brain [(11)C]carfentanil binding following tobacco smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2015;59:100–04.
Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, et al. Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry. 2005;57:1573–82.
Weerts EM, Wand GS, Kuwabara H, Munro CA, Dannals RF, Hilton J, et al. Positron emission tomography imaging of mu- and delta-opioid receptor binding in alcohol-dependent and healthy control subjects. Alcohol Clin Exp Res. 2011;35:2162–73.
Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66:1124–34.
Delfs JM, Kong H, Mestek A, Chen Y, Yu L, Reisine T, et al. Expression of mu opioid receptor mRNA in rat brain: an in situ hybridization study at the single cell level. J Comp Neurol. 1994;345:46–68.
Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402.
Newman S, Pascal L, Sadeghian K, Baldo BA. Sweetened-fat intake sensitizes gamma-aminobutyric acid-mediated feeding responses elicited from the nucleus accumbens shell. Biol Psychiatry. 2013;73:843–50.
Gaykema RP, Nguyen XM, Boehret JM, Lambeth PS, Joy-Gaba J, Warthen DM, et al. Characterization of excitatory and inhibitory neuron activation in the mouse medial prefrontal cortex following palatable food ingestion and food driven exploratory behavior. Front Neuroanat. 2014;8:60.
Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34:50–72.
Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–19.
Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, et al. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology. 2012;219:401–10.
Gass JT, Chandler LJ. The plasticity of extinction: contribution of the prefrontal cortex in treating addiction through inhibitory learning. Front Psychiatry. 2013;4:46.
Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28:6046–53.
Rhodes SE, Killcross AS. Lesions of rat infralimbic cortex result in disrupted retardation but normal summation test performance following training on a Pavlovian conditioned inhibition procedure. Eur J Neurosci. 2007;26:2654–60.
Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–88.
Norgren R, Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Res. 1975;92:123–9.
Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol. 1998;399:440–68.
Schier LA, Hashimoto K, Bales MB, Blonde GD, Spector AC. High-resolution lesion-mapping strategy links a hot spot in rat insular cortex with impaired expression of taste aversion learning. Proc Natl Acad Sci USA. 2014;111:1162–7.
Yamamoto T, Matsuo R, Kawamura Y. Localization of cortical gustatory area in rats and its role in taste discrimination. J Neurophysiol. 1980;44:440–55.
Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–28.
Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. II. Inf Process Tast Qual J Neurophysiol. 1985;53:1356–69.
Katz DB, Simon SA, Nicolelis MA. Taste-specific neuronal ensembles in the gustatory cortex of awake rats. J Neurosci. 2002;22:1850–7.
Chen X, Gabitto M, Peng Y, Ryba NJ, Zuker CS. A gustotopic map of taste qualities in the mammalian brain. Science 2011;333:1262–6.
Samuelsen CL, Fontanini A. Processing of intraoral olfactory and gustatory signals in the gustatory cortex of awake rats. J Neurosci. 2017;37:244–57.
Baldo BA, Spencer RC, Sadeghian K, Mena JD. GABA-mediated inactivation of medial prefrontal and agranular insular cortex in the rat: contrasting effects on hunger- and palatability-driven feeding. Neuropsychopharmacology 2016;41:960–70.
Ferezou I, Hill EL, Cauli B, Gibelin N, Kaneko T, Rossier J, et al. Extensive overlap of mu-opioid and nicotinic sensitivity in cortical interneurons. Cereb Cortex. 2007;17:1948–57.
Lau BK, Ambrose BP, Thomas CS, Qiao M, Borgland SL. Mu-opioids suppress GABAergic synaptic transmission onto orbitofrontal cortex pyramidal neurons with subregional selectivity. J Neurosci. 2020;40:5894–907.
Taki K, Kaneko T, Mizuno N. A group of cortical interneurons expressing mu-opioid receptor-like immunoreactivity: a double immunofluorescence study in the rat cerebral cortex. Neuroscience. 2000;98:221–31.
Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. J Physiol. 2012;590:715–24.
Krook-Magnuson E, Luu L, Lee SH, Varga C, Soltesz I. Ivy and neurogliaform interneurons are a major target of mu-opioid receptor modulation. J Neurosci. 2011;31:14861–70.
Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–36.
McQuiston AR, Saggau P. Mu-opioid receptors facilitate the propagation of excitatory activity in rat hippocampal area CA1 by disinhibition of all anatomical layers. J Neurophysiol. 2003;90:1936–48.
Pezze M, McGarrity S, Mason R, Fone KC, Bast T. Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. J Neurosci. 2014;34:7931–46.
van Duuren E, van der Plasse G, van der Blom R, Joosten RN, Mulder AB, Pennartz CM, et al. Pharmacological manipulation of neuronal ensemble activity by reverse microdialysis in freely moving rats: a comparative study of the effects of tetrodotoxin, lidocaine, and muscimol. J Pharm Exp Ther. 2007;323:61–9.
Gardner MP, Fontanini A. Encoding and tracking of outcome-specific expectancy in the gustatory cortex of alert rats. J Neurosci. 2014;34:13000–17.
Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;65:654–61.
Avery JA, Liu AG, Ingeholm JE, Gotts SJ, Martin A. Viewing images of foods evokes taste quality-specific activity in gustatory insular cortex. Proc Natl Acad Sci USA. 2021;118. https://doi.org/10.1073/pnas2010932118.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–35.
Sadacca BF, Mukherjee N, Vladusich T, Li JX, Katz DB, Miller P. The behavioral relevance of cortical neural ensemble responses emerges suddenly. J Neurosci. 2016;36:655–69.
Mukherjee N, Wachutka J, Katz DB. Impact of precisely-timed inhibition of gustatory cortex on taste behavior depends on single-trial ensemble dynamics. Elife. 2019;8. https://doi.org/10.7554/elife45968.
Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol Behav. 2011;104:64–8.
Touzani K, Bodnar RJ, Sclafani A. Acquisition of glucose-conditioned flavor preference requires the activation of dopamine D1-like receptors within the medial prefrontal cortex in rats. Neurobiol Learn Mem. 2010;94:214–9.
Malkusz DC, Banakos T, Mohamed A, Vongwattanakit T, Malkusz G, Saeed S, et al. Dopamine signaling in the medial prefrontal cortex and amygdala is required for the acquisition of fructose-conditioned flavor preferences in rats. Behav Brain Res. 2012;233:500–7.
Gomes I, Sierra S, Lueptow L, Gupta A, Gouty S, Margolis EB, et al. Biased signaling by endogenous opioid peptides. Proc Natl Acad Sci USA. 2020;117:11820–28.
Thompson GL, Lane JR, Coudrat T, Sexton PM, Christopoulos A, Canals M. Biased agonism of endogenous opioid peptides at the mu-opioid receptor. Mol Pharm. 2015;88:335–46.
LaVigne J, Keresztes A, Chiem D, Streicher JM. The endomorphin-1/2 and dynorphin-B peptides display biased agonism at the mu opioid receptor. Pharm Rep. 2020;72:465–71.
Mizoguchi H, Tseng LF, Suzuki T, Sora I, Narita M. Differential mechanism of G-protein activation induced by endogenous mu-opioid peptides, endomorphin and beta-endorphin. Jpn J Pharm. 2002;89:229–34.
Mole TB, Irvine MA, Worbe Y, Collins P, Mitchell SP, Bolton S, et al. Impulsivity in disorders of food and drug misuse. Psychol Med. 2015;45:771–82.
Velazquez-Sanchez C, Ferragud A, Moore CF, Everitt BJ, Sabino V, Cottone P. High trait impulsivity predicts food addiction-like behavior in the rat. Neuropsychopharmacology 2014;39:2463–72.
Anastasio NC, Stutz SJ, Price AE, Davis-Reyes BD, Sholler DJ, Ferguson SM, et al. Convergent neural connectivity in motor impulsivity and high-fat food binge-like eating in male Sprague-Dawley rats. Neuropsychopharmacology 2019;44:1752–61.
Acknowledgements
The authors thank Julio Diaz, Alexius Lampkin, and Kate Dunaway for assistance with general laboratory tasks. We also are very grateful to Dr. Matt Andrzejewski for assisting with programming the DRL task. We would like to dedicate this paper to the late Dr. Donata Oertel, eminent neuroscientist and former director of the Physiology Graduate Training Program of UW-Madison. Her passion for mentoring young scientists was extraordinary; we will always remember her generous spirit, and we will always be inspired by the example she set.
Author information
Authors and Affiliations
Contributions
JLG wrote the “Methods” and “Results” with editorial assistance from BAB, assisted in writing the “Introduction” and “Discussion”, assisted in designing the experiments, carried out all experiments with assistance from EKG, RAS, and KS, and conducted all statistical analyses. EKG, RAS, and KS assisted in carrying out all experiments. BAB (PI) conceptualized the study, designed the experiments, wrote the “Abstract”, “Introduction”, and “Discussion” assisted by JLG, and supervised all other authors in their respective tasks.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Giacomini, J.L., Geiduschek, E., Selleck, R.A. et al. Dissociable control of μ-opioid-driven hyperphagia vs. food impulsivity across subregions of medial prefrontal, orbitofrontal, and insular cortex. Neuropsychopharmacol. 46, 1981–1989 (2021). https://doi.org/10.1038/s41386-021-01068-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-021-01068-5
- Springer Nature Switzerland AG
This article is cited by
-
Mesolimbic dopamine release precedes actively sought aversive stimuli in mice
Nature Communications (2023)
-
Delayed estrogen actions diminish food consumption without changing food approach, motor activity, or hypothalamic activation elicited by corticostriatal µ-opioid signaling
Neuropsychopharmacology (2023)
-
Eating driven by the gustatory insula: contrasting regulation by infralimbic vs. prelimbic cortices
Neuropsychopharmacology (2022)
-
Mapping causal generators of appetitive motivation-hedonic functions in frontal cortex
Neuropsychopharmacology (2022)