Abstract
Rationale
Attentional deficits are common symptoms in schizophrenia. Recent evidence suggests that schizophrenic patients show abnormalities in spatial orienting of attention, particularly a deficit of inhibition of return (IOR). IOR is mostly thought to reflect an automatic, inhibitory mechanism protecting the organism from redirecting attention to previously scanned, insignificant locations. Pharmacologic challenges with hallucinogens have been used as models for psychosis.
Objectives
The aim of this study was to investigate the neural correlates underlying orienting of attention in the human N-methyl-d-aspartic acid antagonist and 5-HT2A agonist models of psychosis.
Materials and methods
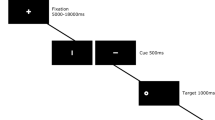
Fourteen healthy volunteers participated in a randomized, double-blind, cross-over event-related functional magnetic resonance imaging (fMRI) study with dimethyltryptamine (DMT) and S-ketamine. We administered a covert orienting of attention task with nonpredictive peripheral cues, and we scanned the subjects on two separate days at least 14 days apart with a placebo and a verum condition on each day.
Results
DMT, but not S-ketamine, slowed down reaction times significantly. IOR was blunted after DMT, but not after S-ketamine. Relative to placebo, S-ketamine increased activation in the IOR condition in the right superior frontal gyrus, left superior temporal gyrus, and right midfrontal frontal gyrus.
Conclusions
The discrepancy between the behavioral and functional imaging outcome indicates that pharmacological fMRI might be a sensitive tool to detect drug-modulated blood oxygenation level-dependent signal changes in the absence of behavioral abnormalities. Our findings might help to further clarify the contradictory findings of IOR in schizophrenic patients and might, thus, shed more light on possible differential pathomechanisms of schizophrenic symptoms.



Similar content being viewed by others
References
Abel KM, Allin MP, Kucharska-Pietura K, Andrew C, Williams S, David AS, Phillips ML (2003) Ketamine and fMRI BOLD signal: distinguishing between effects mediated by change in cognitive state. Hum Brain Mapp 18:135–145
Abi-Saab WM, D’Souza DC, Moghaddam B, Krystal JH (1998) The NMDA antagonist model for schizophrenia: promise and pitfalls. Pharmacopsychiatry 31(Suppl 2):104–109
Addington J, Addington D (1998) Visual attention and symptoms in schizophrenia: a 1-year follow-up. Schizophr Res 34:95–99
American Psychiatric Association (APA) (1994) DSM-IV: diagnostic and statistical manual of mental disorders. American Psychiatric Association, Washington, DC
Andreasen NC (1983) The Scale for the Assessment of Negative Symptoms (SANS). The University of Iowa, Iowa City, IA
Andreasen NC (1984) The Scale for the Assessment of Positive Symptoms (SAPS). The University of Iowa, Iowa City, IA
Arolt V, Teichert HM, Steege D, Lencer R, Heide W (1998) Distinguishing schizophrenic patients from healthy controls by quantitative measurement of eye movement parameters. Biol Psychiatry 44:448–458
Burdett NG, Menon DK, Carpenter TA, Jones JG, Hall LD (1995) Visualisation of changes in regional cerebral blood flow (rCBF) produced by ketamine using long TE gradient-echo sequences: preliminary results. Magn Reson Imaging 13:549–553
Carpenter WT (1999) The schizophrenia ketamine challenge study debate. Biol Psychiatry 46:1081–1091
Carter CS, Robertson LC, Chaderjian MR, Celaya LJ, Nordahl TE (1992) Attentional asymmetry in schizophrenia: controlled and automatic processes. Biol Psychiatry 31:909–918
Carter CS, Robertson LC, Chaderjian MR, O’Shora-Celaya L, Nordahl TE (1994) Attentional asymmetry in schizophrenia: the role of illness subtype and symptomatology. Prog Neuropsychopharmacol Biol Psychiatry 18:661–683
Dean B (2003) The cortical serotonin2a receptor and the pathology of schizophrenia: a likely accomplice. J Neurochem 85:1–13
Dittrich A, von Arx S, Staub S (1985) International study on altered states of consciousness (ISASC). Summary of the results. Ger J Psychol 9:319–339
D’Souza DC, Berman RM, Krystal JH, Charney DS (1999) Symptom provocation studies in psychiatric disorders: scientific value, risks, and future. Biol Psychiatry 46:1060–1080
Fecteau JH, Bell AH, Munoz DP (2004) Neural correlates of the automatic and goal-driven biases in orienting spatial attention. J Neurophysiol 92:1728–1737
Fu CHY, Abel KM, Allin MPG, Gasston D, Costafreda SG, Suckling J, Williams SC, McGuire PK (2005) Effects of ketamine on prefrontal and striatal regions in an overt verbal fluency task: a functional magnetic resonance imaging study. Psychopharmacology 183:92–102
Fuentes LJ, Santiago E (1999) Spatial and semantic inhibitory processing in schizophrenia. Neuropsychology 13:259–270
Fuentes LJ, Boucart M, Alvarez R, Vivas AB, Zimmerman MA (1999) Inhibitory processing in visuospatial attention in healthy adults and schizophrenic patients. Schizophr Res 40:75–80
Gold JM, Randolph C, Coppola R, Carpenter CJ, Goldberg TE, Weinberger DR (1992) Visual orienting in schizophrenia. Schizophr Res 7:203–209
Gouzoulis-Mayfrank E, Hermle L, Thelen B, Sass H (1998) History, rationale and potential of experimental hallucinogenic drug research in psychiatry. Pharmacopsychiatry 31(S2):63–68
Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar KA, Büll U, Sass H (1999) Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology 20:565–581
Gouzoulis-Mayfrank E, Heekeren K, Voss T, Moerth D, Thelen B, Meincke U (2004) Blunted inhibion of return (IOR)—a trait marker of schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry 28:389–396
Gouzoulis-Mayfrank E, Heekeren K, Timmerbeil A, Stoll M, Stock C, Obradovic M, Kovar K-A (2005) Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry 38:301–311
Gouzoulis-Mayfrank E, Arnold S, Heekeren K (2006a) Deficient inhibition of return in schizophrenia—further evidence from an independent sample. Prog Neuropsychopharmacol Biol Psychiatry 30:42–49
Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Daumann J, Obradovic M, Kovar KA (2006b) Inhibition of return in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Neuropsychopharmacology 31:431–441
Gouzoulis-Mayfrank E, Balke M, Hajsamou S, Ruhrmann S, Schultze-Luther F, Daumann J, Heekeren K (2007) Orienting of attention in unmedicated patients with schizophrenia, prodromal subjects and healthy relatives. Schizophr Res 97:35–42
Grunwald T, Beck H, Lehnertz K, Blümcke I, Pezer N, Kurthen M, Fernandez G, Van Roost D, Heinze HJ, Kutas M, Elger CE (1999) Evidence relating human verbal memory to hippocampal N-methyl-d-aspartate receptors. Proc Natl Acad Sci U S A 96:12085–12089
Harrison PJ, Weinberger DR (2005) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10:40–68
Holzman PS (1985) Eye movement dysfunctions and psychosis. Int Rev Neurobiol 27:179–205
Honey GD, Honey RAE, Sharar SR, Turner DC, Pomarol-Clotet E, Kumaran D, Simons JS, Hu X, Rugg MD, Bullmore ET, Fletcher PC (2004) Impairment of specific episodic memory processes by sub-psychotic doses of ketamine: the effects of levels of processing at encoding and of subsequent retrieval task. Psychopharmacology 181:445–457
Honey RAE, Honey GD, O’Loughlin C, Sharar SR, Kumaran D, Bullmore ET, Menon DK, Donovan T, Lupson VC, Bisbrown-Chippendale R, Fletcher PC (2005) Acute ketamine administration alters the brain responses to executive demands in a verbal working memory task: an fMRI study. Neuropsychopharmacology 29:1203–1214
Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Hoschl C (2006) Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 20:389–409
Huey ED, Wexler BE (1994) Abnormalities in rapid, automatic aspects of attention in schizophrenia: blunted inhibition of return. Schizophr Res 14:57–63
Hulshoff Pol HE, Schnack HG, Mandl RC, Cahn W, Collins DL, Evans AC, Kahn RS (2004) Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. NeuroImage 21:27–35
Javitt DC, Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148:1301–1308
Kapur S, Seeman P (2002) NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors—implications for models of schizophrenia. Mol Psychiatry 7:837–844
Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmüller B (1980) Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett 20:379–382
Klein RM (2000) Inhibition of return. Trends Cogn Sci 4:138–147
Konradi C, Heckers S (2003) Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther 97:153–179
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humansPsychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Larrison-Faucher A, Briand KA, Sereno AB (2002) Delayed onset of inhibition of return in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 26:505–512
Lepsien J, Pollmann S (2002) Covert reorienting and inhibition of return: an event-related fMRI study. J Cogn Neurosci 14:127–144
Liotti M, Dazzi S, Umilta C (1993) Deficits of the automatic orienting of attention in schizophrenic patients. J Psychiatr Res 27:119–130
Maruff P, Danckert J, Pantelis C, Currie J (1998) Saccadic and attentional abnormalities in patients with schizophrenia. Psychol Med 28:1091–1100
Mayer AR, Dorflinger JM, Rao SM, Seidenberg M (2004a) Neural networks underlying endogenous and exogenous visual-spatial orienting. NeuroImage 23:534–541
Mayer AR, Seidenberg M, Dorflinger JM, Rao SM (2004b) An event-related fMRI study of exogenous orienting: supporting evidence for the cortical basis of inhibition of return? J Cogn Neurosci 16:1262–1271
Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF (2001) Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 158:1809–1817
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Müller NG, Kleinschmidt A (2007) Temporal dynamics of the attentional spolight: neuronal correlates of attentional capture and inhibition of return in early visual cortex. J Cogn Neurosci 19:587–593
Northoff G, Richter A, Bermpohl F, Grimm S, Martin E, Marcar VL, Wahl C, Hell D, Boeker H (2005) NMDA hypofunction in the posterior cingulate as a model for schizophrenia: an exploratory ketamine administration study in fMRI. Schizophr Res 72:235–248
Oie M, Rund BR, Sundet K (1998) Covert visual attention in patients with early-onset schizophrenia. Schizophr Res 34:195–205
Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812
Øye N, Hustveit O, Moberg ER, Pausen O, Skoglund LA (1991) The chiral forms of ketamine as probes for NMDA receptor function in humans. In: Kameyama T, Nabeshima T, Domino ES (eds) NMDA receptor related agents: biochemistry, pharmacology and behavior. Ann Arbor, NPP Books, pp 381–389
Øye N, Paulsen O, Maurset A (1992) Effects of ketamine on sensory perception: evidence for a roel of N-methyl-d-aspartate receptors. J Pharmacol Exp Ther 260:1209–1213
Posner MI, Cohen Y (1984) Components of visual orienting. In: Bouma H, Bouwhuis DG (eds) Attention and performance. Control of language processing. Erlbaum, London, pp 531–556
Posner MI, Early TS, Reiman E, Pardo PJ, Dhawan M (1988) Asymmetries in hemispheric control of attention in schizophrenia. Arch Gen Psychiatry 45:814–821
Rafal RD, Calabresi PA, Brennan CW, Sciolto TK (1989) Saccade preparation inhibits reorienting to recently attended locations. J Exp Psychol Hum Percept Perform 15(4):673–685
Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE (2004) Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry 161:1004–1015
Rosen AC, Rao SM, Caffarra P, Scaglioni A, Bobholz JA, Woodley SJ, Hammeke TA, Cunningham JM, Prieto TE, Binder JR (1999) Neural basis of endogenous and exogenous spatial orienting. A functional MRI study. J Cogn Neurosci 11:135–152
Sapir A, Soroker N, Berger A, Henik A (1999) Inhibition of return in spatial attention: direct evidence for collicular generation. Nat Neurosci 2:1053–1054
Sapir A, Henik A, Dobrusin M, Hochman EY (2001) Attentional asymmetry in schizophrenia: disengagement and inhibition of return deficits. Neuropsychology 15:361–370
Schlosser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P (2003) Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. NeuroImage 19:751–763
Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkotter J (2007) Schizophrenia proneness instrument, adult version (SPI-A). Giovanni Fioriti Editore s.r.l, Rome, Italy
Sereno AB, Holzman PS (1995) Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry 37:394–401
Snyder SH (1988) Psychotogenic drugs as models for schizophrenia. Neuropsychopharmacology 1:197–199
Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R (1994) Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry 51:98–108
Strauss ME, Novakovic T, Tien AY, Bylsma F, Pearlson GD (1991) Disengagement of attention in schizophrenia. Schizophr Res 37:139–146
Strauss ME, Alphs L, Boekamp J (1992) Disengagement of attention in chronic schizophrenia. Psychiatry Res 43:87–92
Talairach J, Tournaoux P (1988) Co-planar stereotactic atlas of the human brain. Thieme, Stuttgart
Tsai G, Coyle JT (2002) Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 42:165–179
Vollenweider FX, Antonini A, Leenders KL, Oye I, Hell D, Angst J (1997) Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol 7:25–38
Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL (2000) Effects of (S)-ketamine on striatal dopamine: a [11C]raclopride PET study of a model psychosis in humans. J Psychiatr Res 34:35–43
Weinberger DR, Berman KF, Zec RF (1986) Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 43:114–124
Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE (2001) Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 50:825–844
Acknowledgements
This work was supported by a grant to E. Gouzoulis-Mayfrank from the German Research Foundation (Deutsche Forschungsgemeinschaft DFG, Project No. 6 of a DFG clinical researcher group KFO 112/1/-1, Go 717/5-1).
Financial disclosures
All authors certify that there is no actual or potential financial interest in relation to the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daumann, J., Heekeren, K., Neukirch, A. et al. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology 200, 573–583 (2008). https://doi.org/10.1007/s00213-008-1237-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1237-1