Abstract
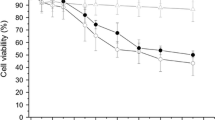
Engineered amorphous silica nanoparticles (nanosilica) are one of the most abundant nanomaterials and are widely used in industry. Furthermore, novel nanosilica materials are promising theranostic tools for biomedicine. However, hazardous effects of nanosilica especially after inhalation into the lung have been documented. Therefore, the safe development of nanosilica materials urgently requires predictive assays to monitor toxicity. Here, we further investigate the impact of the protein corona on the biological activity of two different types of nanosilica (colloidal and pyrogenic) in lung cells. As previously described, adsorption of serum proteins to the nanosilica surface suppresses cytotoxicity in macrophages and lung epithelial cells. As the increase of pro-inflammatory mediators is a hallmark of inflammation in the lung upon nanosilica exposure, we studied the potential coupling of the cytotoxic and pro-inflammatory response in A549 human lung epithelial cells and RAW264.7 mouse macrophages. Indeed, cytotoxicity precedes the onset of pro-inflammatory gene expression and cytokine release as exemplified for IL-8 in A549 cells and TNF-alpha in RAW264.7 macrophages after exposure to 0–100 µg/mL nanosilica in medium without serum. Formation of a protein corona not only inhibited cellular toxicity, but also the pro-inflammatory response. Of note, uptake of nanosilica into cells was negligible in the absence, but enhanced in the presence of a protein corona. Hence, the prevailing explanation that the protein corona simply interferes with cellular uptake thus preventing adverse effects needs to be revisited. In conclusion, for the reliable prediction of adverse effects of nanosilica in the lung, in vitro assays should be performed in media not complemented with complete serum. However, in case of different exposure routes, e.g., injection into the blood stream as intended for biomedicine, the protein corona prevents acute toxic actions of nanosilica.






Similar content being viewed by others
References
Al-Rawi M, Diabaté S, Weiss C (2011) Uptake and intracellular localization of submicron and nano-sized SiO(2) particles in HeLa cells. Arch Toxicol 85:813–826
Blank F, Rothen-Rutishauser BM, Schurch S, Gehr P (2006) An optimized in vitro model of the respiratory tract wall to study particle cell interactions. J Aerosol Med 19:392–405
Casals E, Pfaller T, Duschl A, Oostingh GJ, Puntes V (2010) Time evolution of the nanoparticle protein corona. ACS Nano 4:3623–3632
Delaval M, Wohlleben W, Landsiedel R, Baeza-Squiban A, Boland S (2017) Assessment of the oxidative potential of nanoparticles by the cytochrome c assay: assay improvement and development of a high-throughput method to predict the toxicity of nanoparticles. Arch Toxicol 91:163–177
Diabaté S, Bergfeldt B, Plaumann D, Übel C, Weiss C (2011) Anti-oxidative and inflammatory responses induced by fly ash particles and carbon black in lung epithelial cells. Anal Bioanal Chem 401:3197–3212
Dilger M, Orasche J, Zimmermann R, Paur HR, Diabaté S, Weiss C (2016) Toxicity of wood smoke particles in human A549 lung epithelial cells: the role of PAHs, soot and zinc. Arch Toxicol 90:3029–3044
Donaldson K, Brown D, Clouter A, Duffin R, MacNee W, Renwick L, Tran L, Stone V (2002) The pulmonary toxicology of ultrafine particles. J Aerosol Med 15:213–220
Fritsch-Decker S, Both T, Mülhopt S, Paur HR, Weiss C, Diabaté S (2011) Regulation of the arachidonic acid mobilization in macrophages by combustion-derived particles. Part Fibre Toxicol 8:23
Fritsch-Decker S, Marquardt C, Stoeger T, Diabaté S, Weiss C (2018) Revisiting the stress paradigm for silica nanoparticles: decoupling of the anti-oxidative defense, pro-inflammatory response and cytotoxicity. Arch Toxicol 92:2163–2174
Gaestel M, Kotlyarov A, Kracht M (2009) Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov 8:480–499
Gebel T, Foth H, Damm G, Freyberger A, Kramer PJ, Lilienblum W, Röhl C, Schupp T, Weiss C, Wollin KM, Hengstler JG (2014) Manufactured nanomaterials: categorization and approaches to hazard assessment. Arch Toxicol 88:2191–2211
Gehrke H, Frühmesser A, Pelka J, Esselen M, Hecht LL, Blank H, Schuchmann HP, Gerthsen D, Marquardt C, Diabaté S, Weiss C, Marko D (2013) In vitro toxicity of amorphous silica nanoparticles in human colon carcinoma cells. Nanotoxicology 7:274–293
Haase A, Dommershausen N, Schulz M, Landsiedel R, Reichardt P, Krause BC, Tentschert J, Luch A (2017) Genotoxicity testing of different surface-functionalized SiO2, ZrO2 and silver nanomaterials in 3D human bronchial models. Arch Toxicol 91:3991–4007
Hansjosten I, Rapp J, Reiner L, Vatter R, Fritsch-Decker S, Peravali R, Palosaari T, Joossens E, Gerloff K, Macko P, Whelan M, Gilliland D, Ojea-Jimenez I, Monopoli MP, Rocks L, Garry D, Dawson K, Röttgermann PJF, Murschhauser A, Rädler JO, Tang SVY, Gooden P, Belinga-Desaunay MA, Khan AO, Briffa S, Guggenheim E, Papadiamantis A, Lynch I, Valsami-Jones E, Diabaté S, Weiss C (2018) Microscopy-based high-throughput assays enable multi-parametric analysis to assess adverse effects of nanomaterials in various cell lines. Arch Toxicol 92:633–649
Herd HL, Malugin A, Ghandehari H (2011) Silica nanoconstruct cellular toleration threshold in vitro. J Control Release 153:40–48
Iler R (1981) The surface chemistry of amorphous synthetic silica - interaction with organic molecules in an aqueous medium. In: Dunnom D (ed) Health effects of synthetic silica particulates, STP38665S. ASTM International, West Conshohocken, pp 3–29
Kokkinopoulou M, Simon J, Landfester K, Mailander V, Lieberwirth I (2017) Visualization of the protein corona: towards a biomolecular understanding of nanoparticle-cell-interactions. Nanoscale 9:8858–8870
Landgraf L, Nordmeyer D, Schmiel P, Gao Q, Ritz S, Gebauer S, Graß S, Diabaté S, Treuel L, Graf C, Rühl E, Landfester K, Mailänder V, Weiss C, Zellner R, Hilger I (2017) Validation of weak biological effects by round robin experiments: cytotoxicity/biocompatibility of SiO2 and polymer nanoparticles in HepG2 cells. Sci Rep 7:4341
Laux P, Tentschert J, Riebeling C, Braeuning A, Creutzenberg O, Epp A, Fessard V, Haas KH, Haase A, Hund-Rinke K, Jakubowski N, Kearns P, Lampen A, Rauscher H, Schoonjans R, Störmer A, Thielmann A, Mühle U, Luch A (2018) Nanomaterials: certain aspects of application, risk assessment and risk communication. Arch Toxicol 92:121–141
Lesniak A, Fenaroli F, Monopoli MP, Aberg C, Dawson KA, Salvati A (2012) Effects of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 6:5845–5857
Lynch I, Weiss C, Valsami-Jones E (2014) A strategy for grouping of nanomaterials based on key physio-chemical descriptors as a basis for safer-by-design NMs. Nano Today 9:266–270
Mane SR, Hsiao IL, Takamiya M, Le D, Straehle U, Barner-Kowollik C, Weiss C, Delaittre G (2018) Intrinsically fluorescent, stealth polypyrazoline nanoparticles with large stokes shift for in vivo imaging. Small 14:e1801571
Marquardt C, Fritsch-Decker S, Al-Rawi M, Diabaté S, Weiss C (2017) Autophagy induced by silica nanoparticles protects RAW264.7 macrophages from cell death. Toxicology 379:40–47
Mülhopt S, Diabaté S, Dilger M, Adelhelm C, Anderlohr C, Bergfeldt T, Gomez dlT, Jiang Y, Valsami-Jones E, Langevin D, Lynch I, Mahon E, Nelissen I, Piella J, Puntes V, Ray S, Schneider R, Wilkins T, Weiss C, Paur HR (2018) Characterization of nanoparticle batch-to-batch variability. Nanomaterials (Basel) 8:311
Murugadoss S, Lison D, Godderis L, van den Brule S, Mast J, Brassinne F, Sebaihi N, Hoet PH (2017) Toxicology of silica nanoparticles: an update. Arch Toxicol 91:2967–3010
Neagu M, Piperigkou Z, Karamanou K, Engin AB, Docea AO, Constantin C, Negrei C, Nikitovic D, Tsatsakis A (2017) Protein bio-corona: critical issue in immune nanotoxicology. Arch Toxicol 91:1031–1048
Nowak JS, Mehn D, Nativo P, Garcia CP, Gioria S, Ojea-Jimenez I, Gilliland D, Rossi F (2014) Silica nanoparticle uptake induces survival mechanism in A549 cells by the activation of autophagy but not apoptosis. Toxicol Lett 224:84–92
Nyström AM, Fadeel B (2012) Safety assessment of nanomaterials: implications for nanomedicine. J Control Release 161:403–408
Oh S, Kim B, Kim H (2014) Comparison of nanoparticle exposures between fumed and sol-gel nano-silica manufacturing facilities. Ind Health 52:190–198
Oostingh GJ, Schmittner M, Ehart AK, Tischler U, Duschl A (2008) A high-throughput screening method based on stably transformed human cells was used to determine the immunotoxic effects of fluoranthene and other PAHs. Toxicol In Vitro 22:1301–1310
Panas A, Marquardt C, Nalcaci O, Bockhorn H, Baumann W, Paur HR, Mülhopt S, Diabaté S, Weiss C (2013) Screening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophages. Nanotoxicology 7:259–273
Panas A, Comouth A, Saathoff H, Leisner T, Al-Rawi M, Simon M, Seemann G, Dössel O, Mülhopt S, Paur HR, Fritsch-Decker S, Weiss C, Diabaté S (2014) Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein J Nanotechnol 5:1590–1602
Paur HR, Fissan H, Rothen-Rutishauser B, Teeguarden JG, Diabaté S, Aufderheide M, Kreyling W, Cassee FR, Hänninen O, Kasper G, Riediker M, Schmid O (2011) In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung—a dialogue between aerosol science and biology. J Aerosol Sci 42:668–692
Pavan C, Tomatis M, Ghiazza M, Rabolli V, Bolis V, Lison D, Fubini B (2013) In search of the chemical basis of the hemolytic potential of silicas. Chem Res Toxicol 26:1188–1198
Phalen RF, Oldham MJ, Nel AE (2006) Tracheobronchial particle dose considerations for in vitro toxicology studies. Toxicol Sci 92:126–132
Piret JP, Bondarenko OM, Boyles MSP, Himly M, Ribeiro AR, Benetti F, Smal C, Lima B, Potthoff A, Simion M, Dumortier E, Leite PEC, Balottin LB, Granjeiro JM, Ivask A, Kahru A, Radauer-Preiml I, Tischler U, Duschl A, Saout C, Anguissola S, Haase A, Jacobs A, Nelissen I, Misra SK, Toussaint O (2017) Pan-European inter-laboratory studies on a panel of in vitro cytotoxicity and pro-inflammation assays for nanoparticles. Arch Toxicol 91:2315–2330
Roebuck KA (1999) Regulation of interleukin-8 gene expression. J Interferon Cytokine Res 19:429–438
Ruh H, Kühl B, Brenner-Weiss G, Hopf C, Diabaté S, Weiss C (2012) Identification of serum proteins bound to industrial nanomaterials. Toxicol Lett 208:41–50
Shaw SY, Westly EC, Pittet MJ, Subramanian A, Schreiber SL, Weissleder R (2008) Perturbational profiling of nanomaterial biologic activity. Proc Natl Acad Sci USA 105:7387–7392
Smulders S, Kaiser JP, Zuin S, Van Landuyt KL, Golanski L, Vanoirbeek J, Wick P, Hoet PH (2012) Contamination of nanoparticles by endotoxin: evaluation of different test methods. Part Fibre Toxicol 9:41
Song Y, Li Y, Xu Q, Liu Z (2017) Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: advances, challenges, and outlook. Int J Nanomed 12:87–110
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26:62–69
Stoeger T, Schmid O, Takenaka S, Schulz H (2007) Inflammatory response to TiO2 and carbonaceous particles scales best with BET surface area. Environ Health Perspect 115:A290–A291
van der Zande M, Vandebriel RJ, Groot MJ, Kramer E, Herrera Rivera ZE, Rasmussen K, Ossenkoppele JS, Tromp P, Gremmer ER, Peters RJ, Hendriksen PJ, Marvin HJ, Hoogenboom RL, Peijnenburg AA, Bouwmeester H (2014) Sub-chronic toxicity study in rats orally exposed to nanostructured silica. Part Fibre Toxicol 11:8
Vranic S, Gosens I, Jacobsen NR, Jensen KA, Bokkers B, Kermanizadeh A, Stone V, Baeza-Squiban A, Cassee FR, Tran L, Boland S (2017) Impact of serum as a dispersion agent for in vitro and in vivo toxicological assessments of TiO2 nanoparticles. Arch Toxicol 91:353–363
Wang Y, Kalinina A, Sun T, Nowack B (2016) Probabilistic modeling of the flows and environmental risks of nano-silica. Sci Total Environ 545–546:67–76
Wiemann M, Vennemann A, Sauer UG, Wiench K, Ma-Hock L, Landsiedel R (2016) An in vitro alveolar macrophage assay for predicting the short-term inhalation toxicity of nanomaterials. J Nanobiotechnol 14:16
Winkler HC, Suter M, Naegeli H (2016) Critical review of the safety assessment of nano-structured silica additives in food. J Nanobiotechnol 14:44
Winkler HC, Kornprobst J, Wick P, von Moos LM, Trantakis I, Schraner EM, Bathke B, Hochrein H, Suter M, Naegeli H (2017) MyD88-dependent pro-interleukin-1beta induction in dendritic cells exposed to food-grade synthetic amorphous silica. Part Fibre Toxicol 14:21
Zitvogel L, Kepp O, Kroemer G (2010) Decoding cell death signals in inflammation and immunity. Cell 140:798–804
Acknowledgements
RL and AMW were funded by a grant of the Federal Institute for Risk Assessment (BfR), Germany (BfR-ZEBET-1329-475) which is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leibe, R., Hsiao, IL., Fritsch-Decker, S. et al. The protein corona suppresses the cytotoxic and pro-inflammatory response in lung epithelial cells and macrophages upon exposure to nanosilica. Arch Toxicol 93, 871–885 (2019). https://doi.org/10.1007/s00204-019-02422-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-019-02422-9