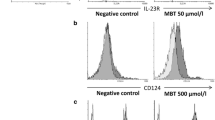
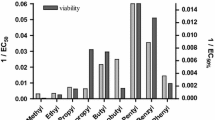
Abstract
The in vitro sensitization assay LCSA (Loose-fit Coculture-based Sensitization Assay) has proved reliable for the detection of contact sensitizers in the past. However, the coculture of human monocyte-derived dendritic cells (DCs) with primary human keratinocytes (KCs) in serum-free medium is relatively complex compared to other sensitization assays which use continuous cell lines. To facilitate high-throughput screening of chemicals, we replaced KCs with the HaCaT cell line under various culture conditions. Coculture of HaCaT with peripheral blood mononuclear cells in serum-supplemented medium leads to generation of CD1a+/CD1c+ DCs after addition of GM-CSF, IL-4, and TGF-β1 (as opposed to CD1a−/CD1c− DCs which arise in the “classic” LCSA coculture). These cells resemble monocyte-derived DCs generated in monoculture, but, unlike those, they show a marked upregulation CD86 after treatment with contact allergens. All of the nine sensitizers in this study were correctly identified by CD1a+/CD1c+ DCs in coculture with HaCaT. Among the substances were weak contact allergens such as propylparaben (which is false negative in the local lymph node assay in mice) and resorcinol (which was not detected by CD1a−/CD1c− DCs in the “classic” LCSA). The level of CD86 upregulation on CD1a+/CD1c+ DCs was higher for most allergens compared to CD1a−/CD1c− DCs, thus improving the assay’s discriminatory power. Three out of four non-sensitizers were also correctly assessed by the coculture assay. A false-positive reaction to caprylic (octanoic) acid confirms earlier results that some fatty acids are able to induce CD86 on DC in vitro. In conclusion, change of the LCSA protocol led to reduction of time and cost while even increasing the assay’s sensitivity and discriminatory power.




Similar content being viewed by others
References
Aeby P, Wyss C, Beck H, Griem P, Scheffler H, Goebel C (2004) Characterization of the sensitizing potential of chemicals by in vitro analysis of dendritic cell activation and skin penetration. J Investig Dermatol 122:1154–1164. doi:10.1111/j.0022-202X.2004.22402.x
Ashby J, Basketter DA, Paton D, Kimber I (1995) Structure activity relationships in skin sensitization using the murine local lymph node assay. Toxicology 103:177–194
Ashikaga T, Yoshida Y, Hirota M, Yoneyama K, Itagaki H, Sakaguchi H, Miyazawa M, Ito Y, Suzuki H, Toyoda H (2006) Development of an in vitro skin sensitization test using human cell lines: the human cell line activation test (h-CLAT). I. optimization of the h-CLAT protocol. Toxicol In Vitro 20:767–773. doi:10.1016/j.tiv.2005.10.012
Basketter DA, Sanders D, Jowsey IR (2007) The skin sensitization potential of resorcinol: experience with the local lymph node assay. Contact Dermat 56:196–200. doi:10.1111/j.1600-0536.2006.01008.x
Basketter DA, Alépée N, Ashikaga T, Barroso J, Gilmour N, Goebel C, Hibatallah J, Hoffmann S, Kern P, Martinozzi-Teissier S, Maxwell G, Reisinger K, Sakaguchi H, Schepky A, Tailhardat M, Templier M (2014) Categorization of chemicals according to their relative human skin sensitizing potency. Dermatitis 25:11–21. doi:10.1097/DER.0000000000000003
Bonifas J, Hennen J, Dierolf D, Kalmes M, Blömeke B (2010a) Evaluation of cytochrome P450 1 (CYP1) and N-acetyltransferase 1 (NAT1) activities in HaCaT cells: implications for the development of in vitro techniques for predictive testing of contact sensitizers. Toxicol In Vitro 24:973–980. doi:10.1016/j.tiv.2009.12.023
Bonifas J, Scheitza S, Clemens J, Blömeke B (2010b) Characterization of N-acetyltransferase 1 activity in human keratinocytes and modulation by para-phenylenediamine. J Pharmacol Exp Ther 334:318–326. doi:10.1124/jpet.110.167874
Boukamp P, Popp S, Altmeyer S, Hülsen A, Fasching C, Cremer T, Fusenig NE (1997) Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCaT. Genes Chromosomes Cancer 19:201–214
Briske-Anderson MJ, Finley JW, Newman SM (1997) The influence of culture time and passage number on the morphological and physiological development of Caco-2 cells. Proc Soc Exp Biol Med 214:248–257
Duperrier K, Eljaafari A, Dezutter-Dambuyant C, Bardin C, Jacquet C, Yoneda K, Schmitt D, Gebuhrer L, Rigal D (2000) Distinct subsets of dendritic cells resembling dermal DCs can be generated in vitro from monocytes, in the presence of different serum supplements. J Immunol Methods 238:119–131
Esquenet M, Swinnen JV, Heyns W, Verhoeven G (1997) LNCaP prostatic adenocarcinoma cells derived from low and high passage numbers display divergent responses not only to androgens but also to retinoids. J Steroid Biochem Mol Biol 62:391–399
Frohwein TA, Sonnenburg A, Zuberbier T, Stahlmann R, Schreiner M (2015) Unsaturated compounds induce up-regulation of CD86 on dendritic cells in the in vitro sensitization assay LCSA. Arch Toxicol. doi:10.1007/s00204-015-1527-4
Gerberick GF, Ryan CA, Kern PS, Schlatter H, Dearman RJ, Kimber I, Patlewicz GY, Basketter DA (2005) Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis 16:157–202
Gibbs S (2009) In vitro irritation models and immune reactions. Skin Pharmacol Physiol 22:103–113. doi:10.1159/000178869
Götz C, Pfeiffer R, Tigges J, Ruwiedel K, Hübenthal U, Merk HF, Krutmann J, Edwards RJ, Abel J, Pease C, Goebel C, Hewitt N, Fritsche E (2012) Xenobiotic metabolism capacities of human skin in comparison with a 3D-epidermis model and keratinocyte-based cell culture as in vitro alternatives for chemical testing: phase II enzymes. Exp Dermatol 21:364–369. doi:10.1111/j.1600-0625.2012.01478.x
Grassi F, Dezutter-Dambuyant C, McIlroy D, Jacquet C, Yoneda K, Imamura S, Boumsell L, Schmitt D, Autran B, Debré P, Hosmalin A (1998) Monocyte-derived dendritic cells have a phenotype comparable to that of dermal dendritic cells and display ultrastructural granules distinct from Birbeck granules. J Leukoc Biol 64:484–493
Hamann D, Yazar K, Hamann CR, Thyssen JP, Lidén C (2014) p-Phenylenediamine and other allergens in hair dye products in the United States: a consumer exposure study. Contact Dermatitis 70:213–218. doi:10.1111/cod.12164
Hennen J, Aeby P, Goebel C, Schettgen T, Oberli A, Kalmes M, Blömeke B (2011) Cross talk between keratinocytes and dendritic cells: impact on the prediction of sensitization. Toxicol Sci 123:501–510. doi:10.1093/toxsci/kfr174
Hiorns LR, Bradshaw TD, Skelton LA, Yu Q, Kelland LR, Leyland-Jones B (2004) Variation in RNA expression and genomic DNA content acquired during cell culture. Br J Cancer 90:476–482. doi:10.1038/sj.bjc.6601405
Mehling A, Eriksson T, Eltze T, Kolle S, Ramirez T, Teubner W, van Ravenzwaay B, Landsiedel R (2012) Non-animal test methods for predicting skin sensitization potentials. Arch Toxicol 86:1273–1295. doi:10.1007/s00204-012-0867-6
OECD (2013) Test no. 439: in vitro skin irritation—reconstructed human epidermis test method. OECD guidelines for the testing of chemicals, section 4. doi: 10.1787/9789264203884-en
Opdyke DL (1981) Monographs on fragrance raw materials. Food Cosmet Toxicol 19:237–254
Peiser M, Wanner R, Kolde G (2004) Human epidermal Langerhans cells differ from monocyte-derived Langerhans cells in CD80 expression and in secretion of IL-12 after CD40 cross-linking. J Leukoc Biol 76:616–622. doi:10.1189/jlb.0703327
Robinson MK, Whittle E, Basketter DA (1999) A two-center study of the development of acute irritation responses to fatty acids. Am J Contact Dermat 10:136–145
Schreiner M, Peiser M, Briechle D, Stahlmann R, Zuberbier T, Wanner R (2007) A loose-fit coculture of activated keratinocytes and dendritic cell-related cells for prediction of sensitizing potential. Allergy 62:1419–1428. doi:10.1111/j.1398-9995.2007.01511.x
Schreiner M, Peiser M, Briechle D, Stahlmann R, Zuberbier T, Wanner R (2008) A new dendritic cell type suitable as sentinel of contact allergens. Toxicology 249:146–152. doi:10.1016/j.tox.2008.04.020
Sonnenburg A, Ahuja V, Schreiner M, Platzek T, Stahlmann R (2012) Assessment of the sensitizing potential of textile disperse dyes and some of their metabolites by the loose-fit coculture-based sensitization assay (LCSA). Arch Toxicol 86:733–740. doi:10.1007/s00204-012-0811-9
Sonnenburg A, Schreiner M, Stahlmann R (2015) Assessment of the sensitizing potency of preservatives with chance of skin contact by the loose-fit coculture-based sensitization assay (LCSA). Arch Toxicol 89:2339–2344. doi:10.1007/s00204-014-1406-4
Søsted H, Rustemeyer T, Gonçalo M, Bruze M, Goossens A, Giménez-Arnau AM, Le Coz CJ, White IR, Diepgen TL, Andersen KE, Agner T, Maibach H, Menné T, Johansen JD (2013) Contact allergy to common ingredients in hair dyes. Contact Dermatitis 69:32–39. doi:10.1111/cod.12077
Thyssen JP, Engkilde K, Lundov MD, Carlsen BC, Menné T, Johansen JD (2010) Temporal trends of preservative allergy in Denmark (1985–2008). Contact Dermatitis 62:102–108. doi:10.1111/j.1600-0536.2009.01668.x
Urbisch D, Mehling A, Guth K, Ramirez T, Honarvar N, Kolle S, Landsiedel R, Jaworska J, Kern PS, Gerberick F, Natsch A, Emter R, Ashikaga T, Miyazawa M, Sakaguchi H (2015) Assessing skin sensitization hazard in mice and men using non-animal test methods. Regul Toxicol Pharmacol 71:337–351. doi:10.1016/j.yrtph.2014.12.008
Wanner R, Sonnenburg A, Quatchadze M, Schreiner M, Peiser M, Zuberbier T, Stahlmann R (2010) Classification of sensitizing and irritative potential in a combined in vitro assay. Toxicol Appl Pharmacol 245:211–218. doi:10.1016/j.taap.2010.02.019
Yamashita S, Segawa R, Satou N, Hiratsuka M, Leonard WJ, Hirasawa N (2013) Induction of thymic stromal lymphopoietin production by nonanoic acid and exacerbation of allergic inflammation in mice. Allergol Int 62:463–471. doi:10.2332/allergolint.13-OA-0552
Acknowledgments
Studies were supported by a grant from the German Federal Ministry of Education and Research (grant no. 31P5924). We are also grateful for a grant of the Sonnenfeld Stiftung, Berlin, providing financial support for the FACS Calibur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The LCSA is patented in Germany with the application number DE102007006736B4 (patent holder: Mrs. Dagmar Briechle). The authors have no financial relationship with the patent holder and declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00204-016-1748-1.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Frombach, J., Sonnenburg, A., Krapohl, BD. et al. A novel method to generate monocyte-derived dendritic cells during coculture with HaCaT facilitates detection of weak contact allergens in cosmetics. Arch Toxicol 91, 339–350 (2017). https://doi.org/10.1007/s00204-016-1722-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1722-y