Abstract
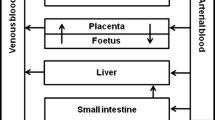
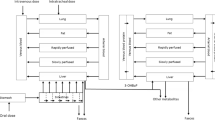
The use of laboratory animals for toxicity testing in chemical safety assessment meets increasing ethical, economic and legislative constraints. The development, validation and application of reliable alternatives for in vivo toxicity testing are therefore urgently needed. In order to use toxicity data obtained from in vitro assays for risk assessment, in vitro concentration–response data need to be translated into in vivo dose–response data that are needed to obtain points of departure for risk assessment, like a benchmark dose (BMD). In the present study, we translated in vitro concentration–response data of the retinoid all-trans-retinoic acid (ATRA), obtained in the differentiation assay of the embryonic stem cell test, into in vivo dose–response data using a physiologically based kinetic model for rat and human that is mainly based on kinetic model parameter values derived using in vitro techniques. The predicted in vivo dose–response data were used for BMD modeling, and the obtained BMDL10 values [lower limit of the 95 % confidence interval on the BMD at which a benchmark response equivalent to a 10 % effect size (BMR10) is reached (BMD10)] for rat were compared with BMDL10 values derived from in vivo developmental toxicity data in rats reported in the literature. The results show that the BMDL10 values from predicted dose–response data differ about sixfold from the BMDL10 values obtained from in vivo data, pointing at the feasibility of using a combined in vitro–in silico approach for defining a point of departure for toxicological risk assessment.






Similar content being viewed by others
Abbreviations
- 3Rs:
-
Replacement, reduction and refinement
- ATRA:
-
All-trans-retinoic acid
- AUC:
-
Area under the concentration–time curve
- BMD:
-
Benchmark dose
- BMDL10 :
-
Lower limit of the 95 % confidence interval on the BMD at which a benchmark response equivalent to a 10 % effect size (BMR10) is reached (BMD10)
- C max :
-
Maximum concentration
- CYP:
-
Cytochrome P450
- MPPGL:
-
Microsomal protein per gram of liver
- P app :
-
Apparent permeability
- PBK:
-
Physiologically based kinetic
- PDMS:
-
Polydimethylsiloxane
- POD:
-
Point of departure
- SPME:
-
Solid-phase microextraction
- UGT:
-
Uridine 5′-diphospho-glucuronosyltransferase
Refer ences
Adamson PC, Pitot HC, Balis FM, Rubin J, Murphy RF, Poplack DG (1993) Variability in the oral bioavailability of all-trans-retinoic acid. J Natl Cancer Inst 85:993–996
Ahmad M, Ahmadi M, Nicholls PJ, Smith HJ (2000) In-vitro metabolism of retinoic acid by different tissues from male rats. J Pharm Pharmacol 52:511–515
Beierschmitt WP, Weiner M (1986) Age-related changes in renal metabolism of acetaminophen in male fisher 344 rats. Age 9:7–13
Blaauboer BJ (2010) Biokinetic modeling and in vitro–in vivo extrapolations. J Toxicol Environ Health B Crit Rev 3:242–252
Bosgra S, van Eijkeren J, Bos P, Zeilmaker M, Slob W (2012) An improved model to predict physiologically based model parameters and their inter-individual variability from anthropometry. Crit Rev Toxicol 42:751–767
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13:407–484
Bürgin H, Schmitt G (2003) Comparison of the embryo-fetal toxicity of all-trans-retinoic acid in two strains of Wistar rat. In: Poster annual meeting European Teratology Society (data provided by Nicole Clemann)
Collins MD, Mao GE (1999) Teratology of retinoids. Annu Rev Pharmacol Toxicol 39:399–430
Collins MD, Tzimas G, Bürgin H, Hummler H, Nau H (1995) Single versus multiple dose administration of all-trans-retinoic acid during organogenesis: differential metabolism and transplacental kinetics in rat and rabbit. Toxicol Appl Pharmacol 130:9–18
Daston GP, Chapin RE, Scialli AR, Piersma AH, Carney EW, Rogers JM, Friedman JM (2010) A different approach to validating screening assays for developmental toxicity. Birth Defects Res B Dev Reprod Toxicol 89:526–530
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
DeJongh J, Nordin-Andersson M, Ploeger BA, Forsby A (1999) Estimation of systemic toxicity of acrylamide by integration of in vitro toxicity data with kinetic simulations. Toxicol Appl Pharmacol 158:261–268
El Mansouri S, Tod M, Leclerq M, Petitjean O, Perret G, Porthault M (1995) Time- and dose-dependent kinetics of all-trans-retinoic acid in rats after oral or intravenous administration(s). Drug Metab Dispos 23:227–231
Forsby A, Blaauboer BJ (2007) Integration of in vitro neurotoxicity data with biokinetic modelling for the estimation of in vivo neurotoxicity. Hum Exp Toxicol 26:333–338
Gülden M, Dierickx P, Seibert H (2006) Validation of a prediction model for estimating serum concentrations of chemicals which are equivalent to toxic concentrations in vitro. Toxicol In Vitro 20:1114–1124
Hosseinpour M, Behdad A (2008) Evaluation of small bowel measurement in alive patients. Surg Radio Anat 30:653–655
Kararli TT (1995) Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16:351–380
Kharasch ED, Walker A, Isoherranen N, Hoffer C, Sheffels P, Thummel K, Whittington D, Ensign D (2007) Influence of CYP3A5 genotype on the pharmacokinetics and pharmacodynamics of the cytochrome P4503A probes alfentanil and midazolam. Clin Pharmacol Ther 82:410–426
Lee HB, Blaufox MD (1985) Blood volume in the rat. J Nucl Med 26:72–76
Li H, van Ravenzwaay B, Rietjens IMCM, Louisse J (2013) Assessment of an in vitro transport model using BeWo b30 cells to predict placental transfer of compounds. Arch Toxicol 87:1661–1669
Lin YS, Dowling ALS, Quigley SD, Farin FM, Zhang J, Lamba J, Schuetz AG, Thummel KE (2002) Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol Pharmacol 62:162–172
Little JM, Lehman PA, Nowell S, Samokyszyn V, Radominska A (1997) Glucuronidation of all-trans-retinoic acid and 5,6-epoxy-all-trans-retinoic acid. Activation of rat liver microsomal UDP-glucuronosyltransferase activity by alamethicin. Drug Metab Dispos 25:5–11
Louisse J, de Jong E, van de Sandt JJM, Blaauboer BJ, Woutersen RA, Piersma AH, Rietjens IMCM, Verwei M (2010a) The use of in vitro toxicity data and physiologically based kinetic modeling to predict dose-response curves for in vivo developmental toxicity of glycol ethers in rat and man. Toxicol Sci 118:470–484
Louisse J, Bai Y, Verwei M, van de Sandt JJM, Blaauboer BJ, Rietjens IMCM (2010b) Decrease of intracellular pH as possible mechanism of developmental toxicity of glycol ether alkoxyacetic acid metabolites. Tox Appl Pharm 245:236–243
Louisse J, Gönen S, Rietjens IMCM, Verwei M (2011) Relative developmental toxicity potencies of retinoids in the embryonic stem cell test compared with their relative potencies in in vivo and two other in vitro assays for developmental toxicity. Toxicol Lett 203:1–8
Louisse J, Verwei M, Woutersen RA, Blaauboer BJ, Rietjens IMCM (2012) Toward in vitro biomarkers for developmental toxicity and their extrapolation to the in vivo situation. Expert Opin Drug Metab Toxicol 8:11–27
Lutz JD, Dixit V, Yeung CK, Dickmann LJ, Zelter A, Thatcher JE, Nelson WL, Isoherranen N (2009) Expression and functional characterization of cytochrome P450 26A1, a retinoic acid hydroxylase. Biochem Pharmacol 77:258–268
McConnell EL, Basit AW, Murdan S (2008) Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in vivo experiments. J Pharm Pharmacol 60:63–70
McNally K, Cotton R, Loizou GD (2011) A workflow for global sensitivity analysis of PBPK models. Front Pharmacol 23:31
Medinsky MA, Leavens TL, Csanady GA, Gargas ML, Bond JA (1994) In vivo metabolism of butadiene by mice and rats: a comparison of physiological model predictions and experimental data. Carcinogenesis 15:1329–1340
Naraharisetti SB, Lin YS, Rieder MJ, Marciante KD, Psaty BM, Thummel KE, Totah RA (2010) Human liver expression of CYP2C8: gender, age, and genotype effects. Drug Metab Dispos 38:889–893
Paini A, Punt A, Viton F, Scholz G, Delatour T, Marin-Kuan M, Schilter B, van Bladeren PJ, Rietjens IMCM (2010) A physiologically based biodynamic (PBBD) model for estragole DNA binding in rat liver based on in vitro kinetic data and estragole DNA adduct formation in primary hepatocytes. Toxicol Appl Pharmacol 245:57–66
Ravindranath V, Anandatheerthavarada HK (1990) Preparation of brain microsomes with cytochrome P450 activity using calcium aggregation method. Anal Biochem 187:310–313
Reilly JA Jr, Forst CF, Quigley EM, Rikkers LF (1990) Gastric emptying of liquids and solids in the portal hypertensive rat. Dig Dis Sci 35:781–786
Rietjens IMCM, Louisse J, Punt A (2011) Tutorial on physiologically based kinetic modeling in molecular nutrition and food research. Mol Nutr Food Res 55:941–956
Rotroff DM, Wetmore BA, Dix DJ, Ferguson SS, Clewell HJ, Houck KA, Lecluyse EL, Andersen ME, Judson RS, Smith CM, Sochaski MA, Kavlock RJ, Boellmann F, Martin MT, Reif DM, Wambaugh JF, Thomas RS (2010) Incorporating human dosimetry and exposure into high-throughput in vitro toxicity screening. Toxicol Sci 117:348–358
Samokyszyn VM, Gall WE, Zawada G, Freyaldenhoven MA, Chen G, Mackenzie PI, Tephly TR, Radominska-Pandya A (2000) 4-Hydroxyretinoic acid, a novel substrate for human liver microsomal UDP-glucuronosyltransferase(s) and recombinant UGT2B7. J Biol Chem 275:6908–6914
Shelley RS, Jun HW, Price JC, Cadwallader DE (1982) Blood level studies of all-trans- and 13-cis-retinoic acids in rats using different formulations. J Pharm Sci 71:904–907
Sim SC, Edwards RJ, Boobis AR, Ingelman-Sundberg M (2005) CYP3A7 protein expression is high in a fraction of adult human livers and partially associated with the CYP3A7*1C allele. Pharmacogenet Genomics 15:625–631
Smith JE, Milch PO, Muto Y, Goodman DS (1973) The plasma transport and metabolism of retinoic acid in the rat. Biochem J 132:821–827
Strikwold M, Spenkelink B, Woutersen RA, Rietjens IMCM, Punt A (2013) Combining in vitro embryotoxicity data with physiologically based kinetic (PBK) modelling to define in vivo dose-response curves for developmental toxicity of phenol in rat and human. Arch Toxicol 87:1709–1723
Sun WM, Houghton LA, Read NW, Grundy DG, Johnson AG (1988) Effect of meal temperature on gastric emptying of liquids in man. Gut 29:302–305
Sun D, Lennernas H, Welage LS, Barnett JL, Landowski CP, Foster D, Fleisher D, Lee KD, Amidon GL (2002) Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 Drugs. Pharm Res 19:1400–1416
Swanson BN, Frolik CA, Zaharevitz DW, Roller PP, Sporn MB (1981) Dose-dependent kinetics of all-trans-retinoic acid in rats. Plasma levels and excretion into bile, urine, and faeces. Biochem Pharmacol 30:107–113
Tay S, Dickmann L, Dixit V, Isoherranen N (2010) A comparison of the roles of peroxisome proliferator-activated receptor and retinoic acid receptor on CYP26 regulation. Mol Pharmacol 77:218–227
Thatcher JE, Zelter A, Isoherranen N (2010) The relative importance of CYP26A1 in hepatic clearance of all-trans retinoic acid. Biochem Pharmacol 80:903–912
Tzimas G, Thiel R, Chahoud I, Nau H (1997) The area under the concentration-time curve of all-trans-retinoic acid is the most suitable pharmacokinetic correlate to the embryotoxicity of this retinoid in the rat. Toxicol Appl Pharmacol 143:436–444
Vaes W (2010) Assay system for determining binding of hydrophobic drugs. Patent no. WO/2010/117276
Van der Jagt K, Munn SJ, Tørsløv J, de Bruijn J (2004) Alternative approaches can reduce the use of test animals under REACH. Report EUR 21405
Verwei M, van Burgsteden JA, Krul CAM, van de Sandt JJM, Freidig AP (2006a) Prediction of in vivo embryotoxic effect levels with a combination of in vitro studies and PBPK modelling. Toxicol Lett 165:79–87
Verwei M, Freidig AP, Havenaar R, Groten JP (2006b) Predicted serum folate concentrations based on in vitro studies and kinetic modeling are consistent with measured folate concentrations in humans. J Nutr 136:3074–3078
Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ 3rd, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS (2012) Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol Sci 125:157–174
Wise LD, Xue D, Winkelmann CT (2010) Micro-computed tomographic evaluation of fetal skeletal changes induced by all-trans-retinoic acid in rats and rabbits. Birth Defects Res B Dev Reprod Toxicol 89:408–417
Wolbold R, Klein K, Burk O, Nüssler AK, Neuhaus P, Eichelbaum M, Schwab M, Zanger UM (2003) Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 38:978–988
Zaya MJ, Hines RN, Stevens JC (2006) Epirubin glucuronidation and UGT2B7 developmental expression. Drug Metabol Dispos 34:2097–2101
Zimmerman CL, Han S, Wiedmann TS (2001) The absorption of retinoic acids from the gastrointestinal tract is dependent upon chemical structure. Cancer Chemother Pharmacol 47:27–33
Acknowledgments
The authors would like to thank Harvey Clewell (The Hamner Institute for Health Sciences, USA) and Joost Westerhout (TNO, The Netherlands) for their contribution to discussions, Wouter Vaes and Jaap Jan Stevenhagen (TNO and TNO Triskelion, The Netherlands) for their help with the SPME studies, and Nicole Clemann (Roche, Switzerland) for providing the in vivo developmental toxicity data of Bürgin and Schmitt (2003). This work was supported by the Netherlands Organization for Health Research and Development (ZonMw; Project No. 114000088). B.J. Blaauboer received financial support from the Doerenkamp-Zbinden Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Louisse, J., Bosgra, S., Blaauboer, B.J. et al. Prediction of in vivo developmental toxicity of all-trans-retinoic acid based on in vitro toxicity data and in silico physiologically based kinetic modeling. Arch Toxicol 89, 1135–1148 (2015). https://doi.org/10.1007/s00204-014-1289-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1289-4