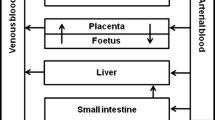
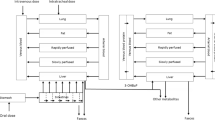
Abstract
In vitro assays are often used for the hazard characterisation of compounds, but their application for quantitative risk assessment purposes is limited. This is because in vitro assays cannot provide a complete in vivo dose–response curve from which a point of departure (PoD) for risk assessment can be derived, like the no observed adverse effect level (NOAEL) or the 95 % lower confidence limit of the benchmark dose (BMDL). To overcome this constraint, the present study combined in vitro data with a physiologically based kinetic (PBK) model applying reverse dosimetry. To this end, embryotoxicity of phenol was evaluated in vitro using the embryonic stem cell test (EST), revealing a concentration-dependent inhibition of differentiation into beating cardiomyocytes. In addition, a PBK model was developed on the basis of in vitro and in silico data and data available from the literature only. After evaluating the PBK model performance, effective concentrations (ECx) obtained with the EST served as an input for in vivo plasma concentrations in the PBK model. Applying PBK-based reverse dosimetry provided in vivo external effective dose levels (EDx) from which an in vivo dose–response curve and a PoD for risk assessment were derived. The predicted PoD lies within the variation of the NOAELs obtained from in vivo developmental toxicity data from the literature. In conclusion, the present study showed that it was possible to accurately predict a PoD for the risk assessment of phenol using in vitro toxicity data combined with reverse PBK modelling.






Similar content being viewed by others
References
Abu-Qare AW, Brownie CF, Abou-Donia MB (2000) Placental transfer and pharmacokinetics of a single oral dose of [14C]p-nitrophenol in rats. Arch Toxicol 74:388–396
Argus (1997) Oral (gavage) developmental toxicity study of phenol in rats. Protocol number: 916-011. Horsham, Pennsylvania
ATSDR (2008) Toxicological profile for phenol. U.S. Department of health and human services, public health service, agency for toxic substances and disease registry (ATSDR), Atlanta (GA)
Baars AJ, Theelen RMC, Janssen PJCM, Hesse JM, van Apeldoorn ME, Meijerink MCM, Verdam L, Zeilmaker MJ (2001) Re-evaluation of human-toxicological maximum permissible risk levels. Report no. 711701025. National Institute for Public Health and the Environment (RIVM), Bilthoven
Barter ZE, Bayliss MK, Beaune PH, Boobis AR, Carlile DJ, Edwards RJ, Houston JB, Lake BG, Lipscomb JC, Pelkonen OR, Tucker GT, Rostami-Hodjegan A (2007) Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human microsomal protein and hepatocellularity per gram of liver. Curr Drug Metab 8:33–45
Berezhkovskiy LM (2004) Volume of distribution at steady state for a linear pharmacokinetic system with peripheral elimination. J Pharm Sci 93:1628–1640
Blacker AM, Schroeder RE, English JC, Murphy SJ, Krasavage WJ, Simon GS (1993) A two-generation reproduction study with hydroquinone in rats. Fundam Appl Toxicol 21:420–424
Bong M, Laskowska-Klita T, Szymczyk T (1985) Effect of the benzene fraction of petroleum on protein content in rat liver and kidney. Bull Environ Contam Toxicol 34:45–54
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13:407–484
Bruce W, Meek ME, Newhook R (2001) Phenol: hazard characterization and exposure-response analysis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 19:305–324
Capel ID, French MR, Millburn P, Smith RL, Williams RT (1972) The fate of (14C)phenol in various species. Xenobiotica 2:25–34
Cassidy MK, Houston JB (1984) In vivo capacity of hepatic and extrahepatic enzymes to conjugate phenol. Drug Metab Dispos 12:619–624
Chapman DE, Namkung MJ, Juchau MR (1994) Benzene and benzene metabolites as embryotoxic agents: effects on cultured rat embryos. Toxicol Appl Pharmacol 128:129–137
Chiu WA, Barton HA, DeWoskin RS, Schlosser P, Thompson CM, Sonawane B, Lipscomb JC, Krishnan K (2007) Evaluation of physiologically based pharmacokinetic models for use in risk assessment. J Appl Toxicol 27:218–237
Chiu WA, Ginsberg GL (2011) Development and evaluation of a harmonized physiologically based pharmacokinetic (PBPK) model for perchloroethylene toxicokinetics in mice, rats, and humans—supplementary materials. Toxicol Appl Pharmacol 253:203–234
Cubitt HE, Houston JB, Galetin A (2009) Relative importance of intestinal and hepatic glucuronidation-impact on the prediction of drug clearance. Pharm Res 26:1073–1083
Cubitt HE, Houston JB, Galetin A (2011) Prediction of human drug clearance by multiple metabolic pathways: integration of hepatic and intestinal microsomal and cytosolic data. Drug Metab Dispos 39:864–873
Delp MD, Evans MV, Duan C (1998) Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J Appl Physiol 85:1813–1822
Dickinson PA, Taylor G (1996) Pulmonary first-pass and steady-state metabolism of phenols. Pharm Res 13:744–748
EC (2007) Corrigendum to regulation (EC) No 1907/2006 of the European parliament and of the council of 18 December 2006 concerning the registration, evaluation, authorisation and restriction of chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Off J Eur Union L 136:3–280
EC (2009) Regulation EC no 1223/2009 of the European parliament and of the council of 30 November 2009 on cosmetic products. Off J Eur Union L 342:59–209
Environment Agency (2009) Contaminants in soil: updated collation of toxicological data and intake values for humans. Phenol. Science Report SC050021/TOX9. Environment Agency, Bristol
Fisher MB, Campanale K, Ackermann BL, Vandenbranden M, Wrighton SA (2000) In vitro glucuronidation using human liver microsomes and the pore-forming peptide alamethicin. Drug Metab Dispos 28:560–566
Genschow E, Spielmann H, Scholz G, Pohl I, Seiler A, Clemann N, Bremer S, Becker K (2004) Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests. Altern Lab Anim 32:209–244.
Gibbs JP, Yang JS, Slattery JT (1998) Comparison of human liver and small intestinal glutathione S-transferase-catalyzed busulfan conjugation in vitro. Drug Metab Dispos 26:52–55
Gray JA, Kavlock RJ (1990) A pharmacokinetic analysis of phenol in the pregnant rat: deposition in the embryo and maternal tissues. Teratology 41:561
Gülden M, Mörchel S, Tahan S, Seibert H (2002) Impact of protein binding on the availability and cytotoxic potency of organochlorine pesticides and chlorophenols in vitro. Toxicology 175:201–213
Hiser MF, Kropscott BE, McGuirk RJ, Bus JS (1994) Pharmacokinetics, metabolism and distribution of 14C-phenol in Fischer 344 rats after gavage, drinking water and inhalation exposure. OTS0557473. Study ID: K-002727-022. Dow Chemical Company. Submitted to U.S. Environmental Protection Agency under TSCA Section 8D
Hughes MF, Hall LL (1995) Disposition of phenol in rat after oral, dermal, intravenous, and intratracheal administration. Xenobiotica 25:873–883
Humphrey MJ, Filer CW, Jeffery DJ, Langley PF, Wadds GA (1980) The availability of carfecillin and its phenol moiety in rat and dog. Xenobiotica 10:771–778
ICRP (2003) Basic anatomical and physiological data for use in radiological protection: reference values. ICRP Publication 89. Pergamon, Oxford
IPCS (2005) Chemical-specific adjustment factors for interspecies differences and human variability: guidance document for use of data in dose/concentration-response assessment. World Health Organization, Geneva
Jones-Price C, Ledoux TA, Reel JR, Fisher P.W., Langhoff-Paschke L, Marr MC, Kimmel CA (1983a) Teratologic evaluation of phenol (CAS No. 108-95-2) in CD rats. NTP Study TER81104. Research Triangle Institute, Research Triangle Park, NC
Jones-Price C, Ledoux TA, Reel JR, Langhoff-Paschke L, Marr MC, Kimmel CA (1983b) Teratologic evaluation of phenol (CAS No. 108-95-2) in CD-1 mice. NTP Study TER80129. Research Triangle Institute, Research Triangle Park, NC
Judis J (1982) Binding of selected phenol derivatives to human serum proteins. J Pharm Sci 71:1145–1147
Kavlock RJ (1990) Structure-activity relationships in the developmental toxicity of substituted phenols: in vivo effects. Teratology 41:43–59
Kheder A, Nair KPS (2012) Spasticity: pathophysiology, evaluation and management. Pract Neurol 12:289–298
Kikuchi K, Itoh Y, Tateoka R, Ezawa A, Murakami K, Niwa T (2010) Metabolomic search for uremic toxins as indicators of the effect of an oral sorbent AST-120 by liquid chromatography/tandem mass spectrometry. J Chromat B Analyt Technol Biomed Life Sci 878:2997–3002
Kothare PA, Zimmerman CL (2002) Intestinal metabolism: the role of enzyme localization in phenol metabolite kinetics. Drug Metab Dispos 30:586–594
Krasavage WJ, Blacker AM, English JC, Murphy SJ (1992) Hydroquinone: a developmental toxicity study in rats. Fundam Appl Toxicol 18:370–375
Landau M (2007) Cardiac complications in deep chemical peels. Dermatol Surg 33:190–193
Liao TF, Oehme FW (1981) Tissue distribution and plasma protein binding of [14C]phenol in rats. Toxicol Appl Pharmacol 57:220–225
Louisse J, de Jong E, van de Sandt JJM, Blaauboer BJ, Woutersen RA, Piersma AH, Rietjens IMCM, Verwei M (2010) The use of in vitro toxicity data and physiologically based kinetic modelling to predict dose-response curves for in vivo developmental toxicity of glycol ethers in rat and man. Toxicol Sci 118:470–484
Luquita MG, Catania VA, Sánchez Pozzi EJ, Veggi LM, Hoffman T, Pellegrino JM, Ikushiro SI, Emi Y, Iyanagi T, Vore M, Mottino AD (2001) Molecular basis of perinatal changes in UDP-glucuronosyltransferase activity in maternal rat liver. J Pharmacol Exp Ther 298:49–56
Medinsky MA, Leavens TL, Csanády GA, Gargas ML, Bond JA (1994) In vivo metabolism of butadiene by mice and rats: a comparison of physiological model predictions and experimental data. Carcinogenesis 15:1329–1340
Murphy SJ, Schroeder RE, Blacker AM, Krasavage WJ, English JC (1992) A study of developmental toxicity of hydroquinone in the rabbit. Fundam Appl Toxicol 19:214–221
Narotsky MG, Kavlock RJ (1995) A multidisciplinary approach to toxicological screening: II Developmental toxicity. J Toxicol Environ Health 45:145–171
Oglesby LA, Ebron-McCoy MT, Logsdon TR, Copeland F, Beyer PE, Kavlock RJ (1992) In vitro embryotoxicity of a series of para-substituted phenols: structure, activity, and correlation with in vivo data. Teratology 45:11–33
Pang KS, Morris ME, Sun H (2008) Formed and preformed metabolites: facts and comparisons. J Pharm Pharmacol 60:1247–1275
Poulin P, Theil FP (2002) Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J Pharm Sci 91:129–156
Powell GM, Miller JJ, Olavesen AH, Curtis CG (1974) Liver as major organ of phenol detoxication? Nature 252:234–235
Punt A, Schiffelers MJWA, Jean Horbach G, van de Sandt JJM, Groothuis GMM, Rietjens IMCM, Blaauboer BJ (2011) Evaluation of research activities and research needs to increase the impact and applicability of alternative testing strategies in risk assessment practice. Regul Toxicol Pharmacol 61:105–114
Rietjens IMCM, Louisse J, Punt A (2011) Tutorial on physiologically based kinetic modeling in molecular nutrition and food research. Mol Nutr Food Res 55:941–956
Rothman N, Bechtold WE, Yin SN, Dosemeci M, Li GL, Wang YZ, Griffith WC, Smith MT, Hayes RB (1998) Urinary excretion of phenol, catechol, hydroquinone, and muconic acid by workers occupationally exposed to benzene. Occup Environ Med 55:705–711
Ryan BM, Selby R, Gingell R, Waechter JM Jr, Butala JH, Dimond SS, Dunn BJ, House R, Morrissey R (2001) Two-generation reproduction study and immunotoxicity screen in rats dosed with phenol via the drinking water. Int J Toxicol 20:121–142
Shirkey RJ, Kao J, Fry JR, Bridges JW (1979) A comparison of xenobiotic metabolism in cells isolated from rat liver and small intestinal mucosa. Biochem Pharmacol 28:1461–1466
Strikwold M, Woutersen RA, Spenkelink B, Punt A, Rietjens IMCM (2012) Relative embryotoxic potency of p-substituted phenols in the embryonic stem cell test (EST) and comparison to their toxic potency in vivo and in the whole embryo culture (WEC) assay. Toxicol Lett 213:235–242
van de Kerkhof EG, de Graaf IAM, Groothuis GMM (2007) In vitro methods to study intestinal drug metabolism. Curr Drug Metab 8:658–675
Verwei M, van Burgsteden JA, Krul CAM, van de Sandt JJM, Freidig AP (2006) Prediction of in vivo embryotoxic effect levels with a combination of in vitro studies and PBPK modelling. Toxicol Lett 165:79–87
Weber M, Weber M (2010) Phenols. In: Pilato L (ed) Phenolic resins: a century of progress. Springer, Berlin, pp 9–23
Weitering JG, Krijgsheld KR, Mulder GJ (1979) The availability of inorganic sulphate as a rate limiting factor in the sulphate conjugation or xenobiotics in the rat? Sulphation and glucuronidation of phenol. Biochem Pharmacol 28:757–762
WHO (1994) Phenol. Environmental health criteria 161. World Health Organization, Geneva
Yamaguchi S (1959) Sulfuric acid esters. I. Synthesis of sulfuric acid esters with sulfamic acid. Nippon Kagaku Zasshi 80:171–173, Chem. Abstr. 27666 (1961)
Zorrilla EP, Inoue K, Fekete ÉM, Tabarin A, Valdez GR, Koob GF (2005) Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol 288:R1450–R1467
Acknowledgments
This study was funded through the programme Alternatives to Animal Experiments III of the Netherlands Organisation for Health Research and Development (ZonMw) (Project Number 114011002).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Strikwold, M., Spenkelink, B., Woutersen, R.A. et al. Combining in vitro embryotoxicity data with physiologically based kinetic (PBK) modelling to define in vivo dose–response curves for developmental toxicity of phenol in rat and human. Arch Toxicol 87, 1709–1723 (2013). https://doi.org/10.1007/s00204-013-1107-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1107-4