Abstract
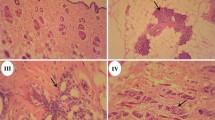
The purpose of this study was to investigate whether the estrogenic effects were detected in the enhanced TG 407 if the estrogenic property was not so strong in the uterotrophic assay. The estrogenic property of 4,4′-(octahydro-4,7-methano-5H-inden-5-ylidene)bisphenol in the uterotrophic assay was slightly stronger than that of genistein or nonylphenol, but weaker than that of ethinyl estradiol. We performed a 28-day repeated-dose toxicity study (enhanced OECD test guideline No. 407) on 4,4′-(octahydro-4,7-methano-5H-inden-5-ylidene)bisphenol based on the OECD draft protocol. The test chemical, administered orally at doses of 0, 10, 50, and 250 mg/kg per day for at least 28 days, caused such estrogenic effects as abnormal estrous cycle, increased ovarian follicles, increased uterine epithelial height, and vaginal mucification in the 50 and/or 250 mg/kg groups. Moreover, follicular epithelial cell hyperplasia of the thyroid was detected in all male rats given the test chemical and in female rats in the 250 mg/kg group. It was concluded that the estrogenic effects were detected in growing rats given 4,4′-(octahydro-4,7-methano-5H-inden-5-ylidene)bisphenol, and thyroid dysfunction was also observed as the endocrine-mediated effects.

Similar content being viewed by others
References
Andrews P, Freyberger A, Hartmann E, Eiben R, Loof I, Schmidt U, Temerowski M, Folkerts A, Stahl B, Kayser M (2002) Sensitive detection of the endocrine effects of the estrogen analogue ethinylestradiol using a modified enhanced subacute rat study protocol (OECD test guideline no. 407). Arch Toxicol 76:194–202
Odum J, Lefevre PA, Tittensor S, Paton D, Routledge EJ, Beresford NA, Sumpter JP, Ashby J (1997) The rodent uterotrophic bioassay: critical protocol features, studies with nonylphenol, and comparison with a yeast estrogenicity assay. Reg Toxicol Pharmacol 25:176–188
OECD (1999) EMSG OECD draft proposal for testing of adequacy of an enhanced OECD protocol, repeated dose (28 days) toxicity (oral) study, based on OECD 407. OECD, Paris
OECD (2003) OECD 4th meeting of the validation management group for mammalian effects testing (VMG-Mammalian) of the task force on endocrine disrupters testing and assessment. OECD, Paris
OECD (2006) OECD 5th meeting of the validation management group for mammalian effects testing (VMG-Mammalian) of the task force on endocrine disrupters testing and assessment. OECD, Washington
OECD (2007a) OECD 6th meeting of the validation management group for mammalian effects testing (VMG-Mammalian) of the task force on endocrine disrupters testing and assessment. OECD, Ljubljana
OECD (2007b) OECD 7th meeting of the validation management group for mammalian effects testing (VMG-Mammalian) of the task force on endocrine disrupters testing and assessment. OECD, Paris
Yamasaki K, Sawaki M, Noda S, Imatanaka M, Takatsuki M (2002a) Subacute oral toxicity study of ethynyl estradiol and bisphenol A based on the draft protocol for the “Enhanced OECD Test Guideline no. 407”. Arch Toxicol 76:65–74
Yamasaki K, Takeyoshi M, Yakabe Y, Sawaki M, Imatanaka M, Takatsuki M (2002b) Comparison of reporter gene assay and immature rat uterotrophic assay of twenty-three chemicals. Toxicology 170:21–30
Yamasaki K, Takeyoshi M, Yakabe Y, Sawaki M, Imatanaka M, Shinoda K, Takatsuki M (2003) Immature rat uterotrophic assay of 18 chemicals and hershberger assay of 30 chemicals. Toxicology 183:95–115
Acknowledgments
This work was supported by a grant from Ministry of Economical Trade and Industry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umano, T., Miyata, K., Minobe, Y. et al. Enhanced OECD TG 407 in detection of endocrine-mediated effects of 4,4′-(octahydro-4,7-methano-5H-inden-5-ylidene)bisphenol. Arch Toxicol 84, 175–182 (2010). https://doi.org/10.1007/s00204-009-0483-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-009-0483-2