Abstract
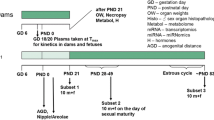
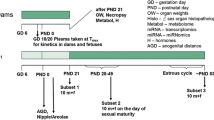
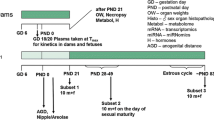
The current investigation examines whether the fungicide vinclozolin, which has an anti-androgenic mode of action, is capable of disrupting endocrine homeostasis at very low doses. The data generated clarify whether a non-monotonic dose–response relationship exists to enhance the current debate about the regulation of endocrine disruptors. Moreover, it is part of a series of investigations assessing the dose–response relationship of single and combined administration of anti-androgenic substances. A pre-postnatal in vivo study design was chosen which was compliant with regulatory testing protocols. The test design was improved by additional endpoints addressing hormone levels, morphology and histopathological examinations. Doses were chosen to represent an effect level (20 mg/kg bw/d), the current NOAEL (4 mg/kg bw/d), and a dose close to the “ADI” (0.005 mg/kg bw/d) for the detection of a possible non-monotonic dose–response curve. Anti-androgenic changes were observable at the effect level but not at lower exposures. Nipple/areola counts appeared to be the most sensitive measure of effect, followed by male sex organ weights at sexual maturation, and finally gross and histopathological findings. The results indicate the absence of evidence for effects at low or very low dose levels. A non-monotonic dose–response relationship was not evident.








Similar content being viewed by others
References
Borgert CJ, Baker SP, Matthews JC (2013) Potency matters, Thresholds govern endocrine activity. Regul Toxicol Pharmacol 67(1):83–88
Carney EW (2004) The effects of feed restriction during in utero and postnatal development in rats. Toxicol Sci 82(1):237–249
Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, Hass U (2010) Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod Toxicol 30(2):313–321
Creasy D, Bube A, de Rijk E, Kandori H, Kuwahara M, Masson R, Nolte T, Reams R, Regan K, Rehm S, Rogerson P, Whitney K (2012) Proliferative and non-proliferative lesions of the rat and mouse male reproductive system. Toxicol Pathol 40(6 Suppl):40S–121S
Delemarre-van de Waal HA, van Coeverden SC, Engelbregt MT (2002) Factors affecting onset of puberty. Horm Res Paediatr 57(2):15–18
Diel P, Schmidt S, Vollmer G, Janning P, Upmeier A, Michna H, Bolt HM, Degen GH (2004) Comparative responses of three rat strains (DA/Han, Sprague-Dawley and Wistar) to treatment with environmental estrogens. Arch Toxicol 78(4):183–193
Fegert I, Billington R, Botham P, Carney E, FitzGerald RE, Hanley T, Lewis R, Marty MS, Schneider S, Sheets LP, Stahl B, van Ravenzwaay B (2012) Feasibility of the extended one-generation reproductive toxicity study (OECD 443). Reprod Toxicol 34(3):331–339
Feuston MH, Bodnar KR, Kerstetter SL, Grink CP, Belcak MJ, Singer EJ (1989) Reproductive toxicity of 2-methoxyethanol applied dermally to occluded and nonoccluded sites in male-rats. Toxicol Appl Pharmacol 100(1):145–161
Food and Agriculture Organization/World Health Organization (1998) Pesticides residues in food—Maximum residue limits, vol 2B, 2nd edn. Codex Alimentarius, Food and Agriculture Organization of the United Nations, Rome
Fussell KC, Schneider S, Buesen R, Groeters S, Strauss V, Melching-Kolmuss S, van Ravenzwaay B (2015) Investigations of putative reproductive toxicity of low-dose exposures to flutamide in Wistar rats. Arch Toxicol 89(12):2385–2402
Goodman JE, Dodge DG, Bailey LA (2010) A framework for assessing causality and adverse effects in humans with a case study of sulfur dioxide. Regul Toxicol Pharmacol 58(2):308–322
Gray LE Jr, Ostby JS, Kelce WR (1994) Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rats. Toxicol Appl Pharmacol 129:46–52
Gray LE, Ostby J, Monosson E, Kelce WR (1999a) Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health 15:48–64
Gray LE, Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J (1999b) Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p '-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health 15:94–118
Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramacheneni D, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L (2001) Effects of environmental antiandrogens on reproductive development in experimental animals. Human Reprod Update 7(3):248–264
Hass U, Christiansen S, Axelstad M, Sorensen KD, Boberg J (2013) Input for the REACH-review in 2013 on endocrine disruptors, Final report, pp. 1–50
Hellwig J, van Ravenzwaay B, Mayer M, Gembardt C (2000) Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Regul Toxicol Pharmacol 32(1):42–50
Holsapple MP, Wallace KB (2008) Dose response considerations in risk assessment—An overview of recent ILSI activities. Toxicol Lett 180:85–92
Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone C, Hotchkiss AK, Gray LE (2008) A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative, dose-additive manner. Toxicol Sci 105(1):153–165
ICH (2005) Study for effects on pre- and postnatal development, including maternal function. In: ICH harmonized tripartite guideline for the detection of toxicity to reproduction for medicinal products and toxicity to male fertility S5(R2) (International conference on harmonization of technical requirements for registration of pharmaceuticals for human use, ed.), pp 8–15
Imperato-McGinley J, Sanchez RS, Spencer JR, Yee B, Vaughan ED (1992) Comparison of the effects of the 5-alpha-reductase inhibitor finasteride and the antiandrogen flutamide on prostate and genital differentiation—dose-response studies. Endocrinology 131:1149–1156
Kavlock R, Cummings R (2005) Mode of action: inhibition of androgen receptor function—Vinclozolin-induced malformations in reproductive development. Crit Rev Toxicol 35(8–9):721–726
Kelce WR, Monosson E, Gamcsik MP, Laws SC, Gray LE (1994) Environmental hormone disruptors: evidence that Vinclozolin developmental toxicity is mediated by anti-androgenic metabolites. Toxicol Appl Pharmacol 126:276–285
Kittel B, Ruehl-Fehlert C, Morawietz G, Klapwijk J, Elwell MR, Lenz B, O’Sullivan MG, Roth DR, Wadsworth PF (2004) Revised guides for organ sampling and trimming in rats and mice - Part 2. Exp Toxicol Pathol 55:413–431
Klimisch HJ, Andreae M, Tillmann U (1997) A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol 25:1–5
Kolle S, Kamp HG, Huener H-A, Knickel J, Verlohner A, Woitkowiak C, Landsiedel R, van Ravenzwaay B (2010) In house validation of a recombinant yeast estrogen and androgen receptor agonist and antagonist screening assays. Toxicol in vitro 24(7):2030–2040
Kortenkamp A, Martin O, Faust M, Evans R, McKinlay R, Orton F, Rosivatz E (2012) State of the art assessment of endocrine disrupters. Final report. (online available)
Laier P, Metzdorff SB, Borch J, Hagen ML, Hass U, Christiansen S, Axelstad M, Kledal T, Dalgaard M, McKinnell C, Brokken LJS, Vinggaard AM (2006) Mechanisms of action underlying the antiandrogenic effects of the fungicide prochloraz. Toxicol Appl Pharmacol 213(2):160–171
Lau C, Klinefelter G, Cameron AM (1996) Reproductive development and functions in the rat after repeated maternal deprivation stress. Toxicol Sci 30(2):298–301
Matsuura I, Saitoh T, Ashina M, Wako Y, Iwata H, Toyota N, Ishizuka Y, Namiki M, Hoshino N, Tsuchitani M (2005) Evaluation of a two-generation reproduction toxicity study adding endpoints to detect endocrine disrupting activity using vinclozolin. J Toxicol Sci 30:163–188
Melching-Kollmuß S, Fussell KC, Buesen R, Dammann M, Schneider S, Tennekes H, van Ravenzwaay B (2014) Anti-androgenicity can only be evaluated using a weight of evidence approach. Regul Toxicol Pharmacol 68(1):175–192
Melching-Kollmuss S, Fussell KC, Schneider S, Buesen R, Groeters S, Strauss V, van Ravenzwaay B (2016) Comparing effect levels of regulatory studies with endpoints derived in targeted anti-androgenic studies: example prochloraz. Arch Toxicol. doi:10.1007/s00204-016-1678-y
Morawietz G, Ruehl-Fehlert C, Kittel B, Bube A, Keane K, Halm S, Heuser A, Hellmann J (2004) Revised guides for organ sampling and trimming in rats and mice–Part 3. Exp Toxicol Pathol 55:433–449
OECD Conceptual Framework (2002). http://www.oecd.org/document/58/0,3343,en_2649_34377_2348794_1_1_1_1,00.html
OECD (2001) OECD Guideline for the testing of chemicals 414: Prenatal developmental toxicity study. OECD 414. 1-22-2001
OECD (2002) Series on testing and assessment no. 21, appraisal of test methods for sex hormone disrupting chemicals, ENV/JM/MONO(2002)8
OECD (2009) Guidance Document for Histologic Evaluation of Endocrine and Reproductive Tests in Rodents. ENV/JM/MONO(2009)11. 28-7-2009
OECD (2011) OECD guideline for the testing of chemicals 443: extended one-generation reproductive toxicity study. OECD 443. 7-28-2011
OECD (2012) Guidance document (GD) on standardized test guidelines for evaluating chemicals for endocrine disruption: case studies using example chemicals. ENV/JM/MONO(2012)34
OECD TG 416 (2001) Two-generation reproduction toxicity study; adopted 22 January 2001
Ostby JS, Gray LE Jr, Kelce WR, Wolf CJ, Huey OP (1997) Sexual differentiation in male rats exposed to low doses of the antiandrogen vinclozolin. Biol Reprod 56(Suppl. 1):99
Rand MN, Breedlove SM (1992) Androgen Locally Regulates Rat Bulbocavernosus and Levator Ani Size. J Neurobiol 23(1):17–30
Rhomberg LR, Goodman JE (2012) Low-dose effects and nonmonotonic dose-responses of endocrine disrupting chemicals: has the case been made? Regul Toxicol Pharmacol 64:130–133
Ruehl-Fehlert C, Kittel B, Morawietz G, Deslex P, Keenan C, Mahrt CR, Nolte T, Robinson M, Stuart BP, Deschl U (2003) Revised guides for organ sampling and trimming in rats and mice—Part 1. Exp Toxicol Pathol 55:91–106
Salewski E (1964) Faerbemethode zum makroskopischen Nachweis von Implantationsstellen am Uterus der Ratte. Naunyn-Schmiedebergs Archiv fuer Experimentelle Pathologie und Pharmakologie 247(4):367
Schneider S, Kaufmann W, Strauss V, van Ravenzwaay B (2011) Vinclozolin: a feasibility and sensitivity study of the ILSI-HESI F1-extended one-generation rat reproduction protocol. Regul Toxicol Pharmacol 59:91–100
Slott VL, Suarez JD, Perreault SD (1991) Rat sperm motility analysis—methodologic considerations. Reprod Toxicol 5(5):449–458
US Environmental Protection Agency (1998a) US EPA Health Effects Test Guideline: OPPTS 870.3700 Prenatal Developmental Toxicity Study
US Environmental Protection Agency (1998b) US EPA Health Effects Test Guideline: OPPTS 870.3800 Reproduction anf Fertility Effects
Van Ravenzwaay B (1992) Discussion of prenatal and reproductive toxicity of Reg. No. 83-258 (vinclozolin). Data submission to U.S. EPA from BASF Corporation, MRID 425813-02
van Ravenzwaay B, Kolle SN, Ramirez T, Kamp HG (2013) Vinclozolin: a case study on the identification of endocrine active substances in the past and a future perspective. Toxicol Lett 223(3):271–279
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455
Wong CI, Kelce WR, Sar M, Wilson EM (1995) Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J Biol Chem 270(34):19998–20003
WHO/IPCS (1998) International programm on chemical safety, Report of IPCS/OECD scoping meeting on endocrine disrupters (EDC´s), Washington
WHO-JMPR (1995) Vinclozolin 375–404
Yamada H, Yamahara A, Yasuda S, Abe M, Oguri K, Fukushima S, Ikeda-Wada S (2002) Dansyl chloride derivatization of methamphetamine: a method with advantages for screening and analysis of methamphetamine in urine. J Anal Toxicol 26:17–22
Zhang F, Rick DL, Kann LH, Perala AW, Geter DR, LeBaron MJ, Bartels MJ (2011) Simultaneous quantitation of testosterone and estradiol in human cell line (H295R) by liquid chromatography/positive atmospheric pressure photoionization tandem mass spectrometry. Rapid Commun Mass Spectrom 25(20):3123–3130
Acknowledgments
The study was (partly) sponsored by CEFIC LRI, 1160 Brussels, Belgium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was co-sponsored by BASF SE, Ludwigshafen, Germany. BASF is producer of vinclozolin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Flick, B., Schneider, S., Melching-Kollmuss, S. et al. Investigations of putative reproductive toxicity of low-dose exposures to vinclozolin in Wistar rats. Arch Toxicol 91, 1941–1956 (2017). https://doi.org/10.1007/s00204-016-1811-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1811-y