Abstract
Summary
Our systematic review and meta-analysis of randomized controlled trials (RCTs) examining whole-body vibration (WBV) effect on bone mineral density (BMD) found significant but small improvements in hip areal BMD (aBMD) in postmenopausal women and in tibia and spine volumetric BMD in children/adolescents, but not in other BMD measurements in postmenopausal women and young adults.
Introduction
Animal experiments report anabolic bone changes in response to WBV, but data in humans are limited. Our objective is to conduct a systematic review and meta-analysis of RCTs examining WBV effect on BMD.
Methods
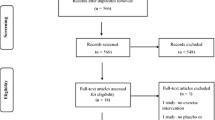
Eligible RCTs included randomized or quasi-randomized trials, with follow-up of ≥6 months, examining WBV effects on BMD in ambulatory individuals without secondary causes of osteoporosis. The weighted mean differences between WBV and control groups in absolute pre-post change in spine and hip aBMD, and in spine and tibia trabecular volumetric BMD (vBMD) were calculated.
Results
Eight RCTs in postmenopausal women (five RCTs), young adults (one RCT), and children and adolescents (two RCTs) were included. The regimens were heterogeneous, study durations were relatively short, and available data was mostly per-protocol. In postmenopausal women, WBV was found to significantly increase hip aBMD (0.015 g cm−2; 95% confidence interval (CI), 0.008–0.022; n = 131) versus controls, but not spine aBMD (n = 181) or tibia trabecular vBMD (n = 29). In young adults, WBV did not increase spine or hip bone mineral content, or tibia trabecular vBMD (n = 53). In children and adolescents, WBV significantly increased spine (6.2 mg cm−3; 95% CI, 2.5–10.0; n = 65) and tibia (14.2 mg cm−3; 95% CI, 5.2–23.2; n = 17) trabecular vBMD.
Conclusions
We found significant but small improvements in BMD in postmenopausal women and children and adolescents, but not in young adults. WBV is a promising new modality, but before recommendations can be made for clinical practice, large-scale long-term studies are needed to determine optimal magnitude, frequency, and duration.





Similar content being viewed by others
References
Eisman JA (2001) Good, good, good... good vibrations: the best option for better bones? Lancet 358:1924–1925
Rubin C, Judex S, Qin YX (2006) Low-level mechanical signals and their potential as a non-pharmacological intervention for osteoporosis. Age Ageing 35:ii32–ii36
Flieger J, Karachalios T, Khaldi L, Raptou P, Lyritis G (1998) Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif Tissue Int 63:510–514
Rubin C, Xu G, Judex S (2001) The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J 15:2225–2229
Rubinacci A, Marenzana M, Cavani F, Colasante F, Villa I, Willnecker J, Moro GL, Spreafico LP, Ferretti MGF, Marrotti G (2008) Ovariectomy sensitizes rat cortical bone to whole-body vibration. Calcif Tissue Int 82:316–326
Judex S, Boyd S, Qin YX, Turner S, Ye K, Muller R, Rubin C (2003) Adaptations of trabecular bone to low magnitude vibrations result in more uniform stress and strain under load. Ann Biomed Eng 31:12–20
Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S (2002) Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone 30:445–452
Rubin C, Turner AS, Muller R, Mittra E, McLeod K, Lin W, Qin YX (2002) Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res 17:349–357
Christiansen BA, Silva MJ (2006) The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann Biomed Eng 34:1149–1156
Judex S, Zhong N, Squire ME, Ye K, Donahue LR, Hadjiargyrou M, Rubin CT (2005) Mechanical modulation of molecular signals which regulate anabolic and catabolic activity in bone tissue. J Cell Biochem 94:982–994
Xie L, Jacobson J, Choi ES, Busa B, Donahue LR, Miller LM, Rubin C, Judex S (2006) Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone 39:1059–1066
Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K (2001) Anabolism. Low mechanical signals strengthen long bones. Nature 412:603–604
Kolata G (2007) Low buzz may give mice better bones and less fat. N Y Times 10:F6
Wikimedia Foundation Inc (2009) Whole-body vibration. In: Wikipedia The Free Encyclopedia. Available via http://en.wikipedia.org/wiki/Whole_body_vibration. Accessed 10 Jul 2009
Kiiski J, Heinonen A, Jarvinen TL, Kannus P, Sievanen H (2008) Transmission of vertical whole body vibration to the human body. J Bone Miner Res 23:1318–1325
Rubin C, Pope M, Fritton JC, Magnusson M, Hansson T, McLeod K (2003) Transmissibility of 15-Hertz to 35-Hertz vibrations to the human hip and lumbar spine: determining the physiologic feasibility of delivering low-level anabolic mechanical stimuli to skeletal regions at greatest risk of fracture because of osteoporosis. Spine 28:2621–2627
Fritton JC, McLeod K, Rubin B (2000) Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech 33:317–325
Prisby RD, Lafage-Proust MH, Malaval L, Belli A, Vico L (2008) Effects of whole-body vibration on the skeleton and other organ systems in man and animal models: what we know and what we need to know. Ageing Res Rev 7:319–329
Higgins J, Green S (2008) Cochrane's handbook for systematic reviews of interventions version 5.0.1. In: The Cochrane Collaboration. Available via http://www.cochrane-handbook.org. Accessed 10 Jul 2009
Moher D, Cook D, Eastwood S, Olkin I, Rennie D, Stroup D (1999) Improving the quality of reports of meta-analyses of randomized controlled trials: the QUOROM statement. Lancet 354:1896–1900
Nelson DA, Norris SA, Gilsanz V (2006) Childhood and adolescence. In: Rosen CJ (ed) Primer on the metabolic bone diseases and disorders of mineral metabolism, 7th edn. American Society for Bone and Mineral Research, Washington, pp 55–63
Reid IR (2006) Menopause. In: Rosen CJ (ed) Primer on the metabolic bone diseases and disorders of mineral metabolism, 7th edn. American Society for Bone and Mineral Research, Washington, pp 68–70
Juni P, Altman G, Egger M (2001) Assessing the quality of controlled clinical trials. Br Med J 323:42–46
Gilsanz V, Wren TAL, Sanchez M, Dorey F, Judex S, Rubin C (2006) Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res 21:1464–1474
Gusi N, Raimundo A, Leal A (2006) Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC Musculoskelet Disord 7:92
Iwamoto J, Takeda T, Sato Y, Uzawa M (2005) Effect of whole-body vibration exercise on lumbar bone mineral density, bone turnover, and chronic back pain in post-menopausal osteoporotic women treated with alendronate. Aging Clin Exp Res 17:157–163
Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, McLeod K (2004) Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 19:343–351
Russo CR, Lauretani F, Bandinelli S, Bartali B, Cavazzini C, Guralnik JM, Ferrucci L (2003) High-frequency vibration training increases muscle power in postmenopausal women. Arch Phys Med Rehabil 84:1854–1857
Torvinen S, Kannus P, Sievanen H, Jarvinen TA, Pasanen M, Kontulainen S, Nenonen A, Jarvinen TL, Paakkala T, Jarvinen M, Vuori I (2003) Effect of 8-month vertical whole body vibration on bone, muscle performance, and body balance: a randomized controlled study. J Bone Miner Res 18:876–884
Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S (2004) Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res 19:352–359
Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z (2004) Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res 19:360–369
Tang B, Eslick G, Nowson C, Smith C, Bensoussan A (2007) Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 370:657–666
Bonnick SL (2004) Changes in bone density. In: Bone densitometry in clinical practice: application and interpretation, 2nd edn. Human Press Inc, Totowa, p 279
Ward KA, Roberts SA, Adams JE, Lanhan-New S, Mughal MZ (2007) Calcium supplementation and weight bearing physical activity—do they have a combined effect on the bone density of pre-pubertal children? Bone 41:496–504
Judex S, Donahue LR, Rubin C (2002) Genetic predisposition to osteoporosis is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J 16:1280–1282
Wolff J (1986) The law of bone remodeling. Springer, Berlin
Ruan XY, Jin FY, Liu Y, Peng ZL, Sundelin YG (2008) Effects of vibration therapy on bone mineral density in postmenopausal women with osteoporosis. Chin Med J 121:1155–1158
Cheung AM, Tile L, Lee Y, Tomlinson G, Hawker G, Scher J, Hu H, Vieth R, Thompson L, Jamal S, Josse R (2008) Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med 5:e196
Martyn-St James M, Carroll S (2006) High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int 17:1225–1240
Acknowledgements
L. Slatkovska is supported by a Canadian Institutes of Health Research/Ontario Women's Health Council doctoral research award; Dr. Shabbir M. H. Alibhai is supported by a Research Scientist Award from the National Cancer Institute of Canada; Dr. AM Cheung is supported by a Canadian Institutes of Health Research/Ontario Women's Health Council Mid-Career Scientist Award. This study was in part supported by the Ontario Premier's Research Excellence award presented to Dr. Angela M. Cheung for innovations in postmenopausal osteoporosis.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Study bias determination
The methodological quality assessment involved identifying the presence of different types of study biases based on the information provided in the included trials (i.e., as published or as reported via email communications). The following types of study biases were identified using an invalidated checklist:
□Selection bias present (check as present if at least one “no” or “unclear” is present) | |||
Yes | No | Unclear | True random study-arm allocation performed |
Yes | No | Unclear | Concealed study-arm allocation performed |
□Performance bias (check as present if at least one “no” or “unclear” is present) | |||
Groups were matched baseline based on minor confounders | |||
Yes | No | Unclear | Calcium intakes |
Yes | No | Unclear | Age at baseline |
Yes | No | Unclear | Menstrual status at baseline |
Yes | No | Unclear | BMD at baseline |
Yes | No | Unclear | Body mass at baseline |
□Detection bias present (check as present if at least one “no” or “unclear” is present) | |||
Yes | No | Unclear | Blinding of those assessing BMD outcomes |
□Attrition bias present (check as present if at least one “no” or “unclear” is present) | |||
Yes | No | Unclear | Only intention-to-treat analysis performed |
Handling of BMD outcomes
Contacting the original authors
We contacted the original authors of all of the included trials to obtain missing information, and we obtained 100% response rate. The mean absolute change in BMD was not reported in a number of original publications [26–30]. After contacting all of the original authors, Iwamoto et al. [26] and Verschueren et al. [30] provided us with the missing mean absolute change in BMD. However, since the original data were no longer available to Rubin et al. [27], Russo et al. [28], and Torvinen et al. [29], some estimations and statistical manipulations were performed to obtain the correct BMD outcome.
Estimations
Where BMD data were reported only as the baseline and final means [28, 29], the mean absolute change in BMD was obtained by subtracting the final from the baseline mean. The standard deviation (SD) corresponding to the absolute BMD change was estimated using the Follmann's method as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [19]. The correlation coefficient value (r) of 0.95 was used in all Follmann's calculations. Based on the 12-month follow-up data collected in our laboratory, we obtained r values ranging from 0.94 to 0.97 for the total hip, femoral neck, and L1–L4 aBMD change in 440 postmenopausal women receiving either placebo or vitamin K treatment [38]. Prior meta-analysis utilized an even higher correlation coefficient value (r = 0.99) in their Follmann's calculations [39]. Therefore, an r of 0.95 seemed to be a conservative value to use in our calculations.
A trial of Rubin et al. [27] required special attention in the determination of the aBMD outcomes. Neither the absolute mean change in aBMD nor the final aBMD was available to us. Instead, baseline aBMD was reported for 56 participants with all follow-up data out of the 70 originally enrolled participants. Also, the mean percent change in aBMD for the ITT (n = 70) and per-protocol data was reported. The mean percent change for the per-protocol data was only reported for different adherence groups but not for the 56 participants with the baseline aBMD. The adherence group with the largest sample size (n = 33) consisted of at least 60% adherent participants. Therefore, for our primary analysis of the per-protocol data, we utilized the baseline aBMD data (n = 56) and the percent change aBMD data (n = 33) to estimate an absolute change in BMD. Alternatively, for our sensitivity analysis of the ITT data, we utilized the baseline BMD data (n = 56) and the percent change aBMD data (n = 70) to estimate an absolute change in aBMD. Finally, the SD corresponding to the mean absolute change in aBMD was imputed from another trial [25] for each study group (i.e., control and vibration) and each measurement site (i.e., L2–L4 and femoral neck). This trial [25] was used, because it is the most similar to the Rubin's et al. [27] trial in terms of its study sample size, population type, and measurement type.
Other statistical manipulations
When CIs [25] or standard errors [28] were reported only, statistical formulas were used to convert these measurements to SDs. When BMD data for more than one arm were pooled into one arm [30], the mean absolute change was obtained using the “weighted mean” formula, and the corresponding SD was obtained using the “pooled or weighted SD” formula. When raw data were provided in the original publication [31] or in our email communication with the original authors [26], appropriate descriptive statistics were used to obtain the mean absolute change and the corresponding SD.
Rights and permissions
About this article
Cite this article
Slatkovska, L., Alibhai, S.M.H., Beyene, J. et al. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int 21, 1969–1980 (2010). https://doi.org/10.1007/s00198-010-1228-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1228-z