Abstract
Purpose
The driving pressure of the respiratory system has been shown to strongly correlate with mortality in a recent large retrospective ARDSnet study. Respiratory system driving pressure [plateau pressure−positive end-expiratory pressure (PEEP)] does not account for variable chest wall compliance. Esophageal manometry can be utilized to determine transpulmonary driving pressure. We have examined the relationships between respiratory system and transpulmonary driving pressure, pulmonary mechanics and 28-day mortality.
Methods
Fifty-six patients from a previous study were analyzed to compare PEEP titration to maintain positive transpulmonary end-expiratory pressure to a control protocol. Respiratory system and transpulmonary driving pressures and pulmonary mechanics were examined at baseline, 5 min and 24 h. Analysis of variance and linear regression were used to compare 28 day survivors versus non-survivors and the intervention group versus the control group, respectively.
Results
At baseline and 5 min there was no difference in respiratory system or transpulmonary driving pressure. By 24 h, survivors had lower respiratory system and transpulmonary driving pressures. Similarly, by 24 h the intervention group had lower transpulmonary driving pressure. This decrease was explained by improved elastance and increased PEEP.
Conclusions
The results suggest that utilizing PEEP titration to target positive transpulmonary pressure via esophageal manometry causes both improved elastance and driving pressures. Treatment strategies leading to decreased respiratory system and transpulmonary driving pressure at 24 h may be associated with improved 28 day mortality. Studies to clarify the role of respiratory system and transpulmonary driving pressures as a prognosticator and bedside ventilator target are warranted.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) [1, 2] carries a high morbidity and mortality and remains a very common clinical problem [3, 4]. The backbone of current treatment is the use of “lung protective” ventilation, i.e. limiting tidal volumes (VT) and keeping end-inspiratory plateau pressures low while maintaining sufficiently high positive end-expiratory pressure (PEEP) [5–9]. Lung protective ventilation strategies are thought to reduce mechanical stress by maintaining alveolar aeration (limiting repetitive opening and closing) while preventing overexpansion of the lung, thereby decreasing ventilator-induced lung injury [10–12]. These strategies have been shown to reduce mortality, demonstrating the importance of respiratory mechanics in determining outcomes in patients with ARDS [5–8].
Results from a recent study using data from nine randomized trials suggest that the driving pressure (DP) of the respiratory system (DPRS), which is easily measured at the bedside (DPRS = plateau pressure − PEEP), may be a superior marker for the severity of lung injury, providing improved prognostication and correlation with mortality [13]. The authors of this study reported that higher DPRS correlated with increased mortality even in patients already receiving low-volume lung protective ventilation [13]; however, they did not account for the effects of the chest wall in their analysis [14]. This latter parameter can be obtained using the transpulmonary DP (DPL = end-inspiratory transpulmonary pressure−end-expiratory transpulmonary pressure), which is the pressure actually applied to the lungs. Using DPL for monitoring and prognostication of ARDS eliminates the variable effects of the chest wall on the respiratory system. In addition, because chest wall compliance and pleural pressure vary widely between patients [15], measuring DPL instead of DPRS may be the more appropriate measure.
Esophageal manometry provides a useful estimate of pleural pressure and can be used to determine separate contributions of lung and chest wall in determining respiratory system mechanics [15–17]. In the EPVent trial, our group tested the use of esophageal manometry for managing mechanical ventilation in patients with ARDS [15]. We found that maintenance of positive transpulmonary pressures resulted in significantly higher PEEP, improved oxygenation (P/F ratio) and improved respiratory system compliance [15]. Maintenance of positive transpulmonary pressures is in accordance with the concept of personalization and “a la carte” ventilatory management in ARDS [18]. In the study reported here, we used esophageal pressure measurements from EPVent to follow changes in DPRS and DPL over time in the two treatment groups.
Methods
Study cohort
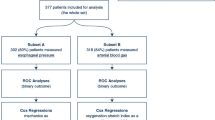
The EPvent study was approved by the institutional review board at the Beth Israel Deaconess Medical Center in Boston, and written consent was obtained from each patient or surrogate [15]. Patients were included in the study if they had acute lung injury or ARDS as defined by the American–European Consensus Conference [2]. Of the original 61 subjects, 56 had sufficient data at baseline and 24 h to calculate DPRS and DPL for further analysis. Of the five patients excluded, three were from the intervention group (1 in the 3 died), and two were from the control group (both died). The full results of the EPVent trial have been published elsewhere [15].
Physiologic measurements
Subjects were monitored while supine with the head of the bed elevated to 30°. An esophageal balloon-catheter was placed, and measurements were obtained to estimate intrathoracic pressures. Airway pressure, tidal volume and air flow were recorded during tidal ventilation and during end-expiratory holds and end-inspiratory holds (plateau) (Fig. 1). Between baseline and 5 min, every patient underwent a recruitment maneuver with an airway pressure increase to 40 cmH2O for 30 s and VT set at 6 cc/kg ideal body weight. Patients in the intervention group had PEEP levels adjusted to achieve a positive transpulmonary pressure of 0–10 cm H2O at end-expiration according to a sliding scale based on the fraction of inspired oxygen (see Fig. 1 in EPvent [15]). The control group had PEEP titrated as per the standard low PEEP ARDSnet tables [5]. Measured variables included total PEEP (measured at end-expiratory hold), plateau pressure, end-expiratory esophageal pressure, end-inspiratory esophageal pressure and VT; all other variables in our study were calculated from these values (Fig. 1). Transpulmonary pressure was calculated as airway pressure minus esophageal pressure (a surrogate for intrathoracic pressure) (Fig. 1). DPRS was calculated as the plateau pressure minus total PEEP (Fig. 1). DPL was calculated from the transpulmonary pressures at the same times (Fig. 1). Elastance of the respiratory system (ERS) was calculated as the change in airway pressure from end-expiratory hold to plateau divided by VT, and lung elastance (EL) was calculated as the change in transpulmonary pressure at the same times. The 28 day mortality was also recorded. Data were analyzed at baseline, at 5 min (after the recruitment maneuver and adjustment of tidal volume and PEEP to protocol settings) and at 24 h.
Pressure and volume tracings from a study patient. a Time tracings show the pressure and volume change during tidal breathing with expiratory and inspiratory holds. Airway pressure (Pao) is the total respiratory system pressure, esophageal pressure (Pes) is an estimate of the pleural and trans-chest wall pressure and transpulmonary pressure (PL) is calculated as Pao minus Pes. DP RS Respiratory system driving pressure [plateau pressure−total positive end-expiratory pressure (PEEP)], DP CW chest wall driving pressure (Pes at end-inspiratory hold−Pes at end-expiratory hold), DP L transpulmonary driving pressure (PL at the end-inspiratory hold−PL at end-expiratory hold). b Pressure–volume (P–V) curves during tidal breathing following respiratory system pressures (Pao), transpulmonary pressures (PL) and chest wall pressures (Pes). Dotted lines represent the static compliance of the respiratory system and lung as measured by the slope between end-inspiratory holds and end-expiratory holds (stars), arrows indicate the direction of the inspiration and expiration
Statistical analysis
Continuous variables with a normal distribution were analyzed via analysis of variance (ANOVA) and linear regression. We compared DPRS and DPL between 28 day survivors and 28 day non-survivors, and compared DPRS, DPL, ERS, EL and PEEP between the control and intervention groups at baseline, 5 min and 24 h. Changes in these variables (DPRS, DPL, etc.) over time between the control and intervention groups and between survivors and non-survivors were assessed by ANOVA repeated measures analysis with the interaction terms (time × physiological measurement) added into the analysis. We used LOWESS (locally weighted scatterplot smoothing) to compare individual values of DPRS and DPL in order to determine if DPRS is predictive of DPL in a given patient and if variation of ∆DPRS resulting from a change in PEEP setting is predictive of the variation of ∆DPL. Dichotomous and nominal variables were compared using chi-square analysis with Fisher’s exact test.
Results
Data from 29 patients in the control group and 27 patients in the intervention group were analyzed. The cohorts were well matched by age, sex, race, Acute Physiology and Chronic Health Evaluation (APACHE) II score at admission, primary physiologic injury, baseline organ failure, gas exchange (pH, PaO2, pCO2), lactate and hemodynamics (Table 1). There were 42 survivors and 14 non-survivors at 28 days. Compared with non-survivors, survivors had a significantly lower APACHE II score (24.7 vs. 31.5; p < 0.0001), higher pH (7.35 vs. 7.27, p < 0.001), lower lactate level (2.1 vs. 5.8, p < 0.0001), but they were otherwise similar in race, gender, age, heart rate and blood pressure at baseline.
To evaluate the correlation between driving pressure and survival we compared 28 day survivors and non-survivors. There was no difference between these groups in baseline DPRS (13.6 vs. 15.5 cmH2O; p = 0.08), baseline DPL (10.1 vs. 10.4 cmH2O; p = 0.75), 5 min DPRS (12.3 vs. 14.7 cmH2O; p = 0.054) or 5 min DPL (8.5 vs. 10.6 cmH2O; p = 0.09), although mean DPL and DPRS were higher in non-survivors at all time points (Fig. 2a, b). At 24 h, survivors had a significantly lower DPRS (10.5 vs. 14.7 cmH2O; p < 0.0001) and DPL (7.8 vs. 10.1 cmH2O; p = 0.03) (Fig. 2a, b). From baseline to 24 h, survivors showed a significant decrease in both DPRS (∆DPRS −3.29 vs. −0.81 cmH2O; p = 0.03) and DPL (∆DPL −2.3 vs. −0.3 cmH2O; p = 0.04) compared with non-survivors. Similarly, ERS and EL were lower at baseline in survivors (ERS 28.1 vs. 35.3 cmH2O/L, p = 0.02; EL 21.1 vs. 24.3 cmH2O/L, p = 0.3) and decreased over 24 h (ERS 25.2 vs. 34.6 cmH2O/L, p = 0.001; EL 18.6 vs 24.4 cmH2O/L, p = 0.05). In both survivors and non-survivors there was no interaction with time (DPRS, p = 0.12; DPL, p = 0.59). Notably, ten of 29 (34.5 %) patients in the control group and four of 27 (14.8 %) patients in the intervention group died by 28 days (p = 0.085).
a, b Respiratory system and transpulmonary driving pressures in survivors (n = 42) and non-survivors (n = 14) at 28 days. c, d Respiratory system and transpulmonary driving pressures in the control group (n = 29) and the intervention group (n = 27). Data points are means with standard errors at baseline, 5 min and 24 h. p values were assessed by analysis of variance (ANOVA)
To evaluate the effects of PEEP adjustment targeting positive transpulmonary pressure on changes in driving pressure, we compared the control and intervention groups. There was no difference between groups in baseline DPRS (14.0 vs. 14.1 cmH2O; p = 0.97), baseline DPL (10.1 vs. 10.3 cmH2O; p = 0.80), 5 min DPRS (12.2 vs. 13.6 cmH2O; p = 0.22) or 5 min DPL (8.5 vs. 9.5 cmH2O; p = 0.35). At 24 h there was no difference in DPRS between groups (12.0 vs. 11.1 cmH2O; p = 0.31), while DPL was significantly lower in the intervention group (9.4 vs. 7.2 cmH2O; p = 0.02) (Table 2; Fig. 2c, d). In terms of changes between baseline and 24 h, the intervention group showed a non-significant change in DPRS (∆DPRS −2.39 vs. −2.97 cmH2O, p = 0.57) and a significant decrease in DPL (∆DPL −0.65 vs. −3.07 cmH2O, p = 0.004). There was a strong interaction between time and DPRS (p = 0.015) and DPL (p < 0.001), respectively. The relationship between DPRS and DPL at any given time point and the relationship between the ∆DPRS and ∆DPL (baseline to 5 min and baseline to 24 h) were assessed by LOWESS, revealing a strong linear relationship, but significant variation in DPL and ∆DPL for any given DPRS or ∆DPRS, respectively (Electronic Supplemental Material figure). There was no difference in DPCW at any time point compared by intervention or mortality, and there was wide variability among all patients in both groups (Table 2).
To evaluate the causes of driving pressure changes within individual subjects, changes in elastance, PEEP and V T were examined concurrently. The decrease in DPL at 24 h was explained by a similar decrease in E L over the same period. There was no difference between control and intervention groups in baseline E RS (29.9 vs 29.9 cmH2O/L, p = 0.99), baseline E L (21.7 vs 22.1 cmH2O/L, p = 0.86), 5 min E RS (29.9 vs 32.2 cmH2O/L, p = 0.48) or 5 min E L (21.1 vs 22.9 cmH2O/L, p = 0.58) (Fig. 3). At 24 h the intervention group had a slight decrease in E RS that did not reach statistical significance (29.8 vs 25.2 cmH2O/L, p = 0.07) and significantly lower E L (23.4 vs 16.5 cmH2O/L, p = 0.007) (Table 2; Fig. 3) and greater change from baseline (ΔE RS −0.09 vs −4.75 cmH2O/L, p = 0.01, ΔE L 1.69 vs −5.66 cmH2O/L, p = 0.0002) relative to the control group. There was a strong correlation between baseline-to-24 h ∆DPRS and ΔE RS (r 2 = 0.36, p < 0.0001) and even stronger correlation between ∆DPL and ΔE L (r 2 = 0.65, p < 0.0001, Fig. 4). In contrast, the improved DP at 24 h did not appear to be related to differences in VT between groups (Table 2).
a Change in driving pressure of the respiratory system (∆DP RS ) vs. change in respiratory system elastance (∆R SE ) between baseline and 24 h. b Change in transpulmonary driving pressure (∆DP L ) vs. change in pulmonary elastance (∆EL) between baseline and 24 h. R 2 was calculated by the linear fit model and p was calculated by ANOVA
As PEEP was the only adjusted variable between groups, any differences in DP and elastance should be secondary to differences in PEEP between the control and intervention groups. At baseline, PEEP was the same between the control group and intervention group prior to initiating the protocol (13.0 vs. 12.7 cmH2O; p = 0.79). Targeting positive end-expiratory transpulmonary pressure in the intervention group resulted in increased PEEP at 5 min (12.9 vs. 20.0 cmH2O; p < 0.0001) and 24 h (11.0 vs. 19.3 cmH2O, p < 0.0001) (Table 2; Fig. 3.)
Discussion
The data from our study suggest that utilizing PEEP titration to target positive transpulmonary pressure via esophageal manometry results in both improved elastance and driving pressures. These findings suggest that strategies leading to decreased DP and elastance could be associated with improved 28 day mortality.
Determinations of driving pressure change
The relationship between DP and its determining variables (elastance and VT) was illustrated in our study, with changes in DPL in the intervention group strongly correlating with improvement in lung elastance. This improvement was seen despite a small increase in mean VT in the intervention group relative to the control group by 24 h. These results clearly suggest that adjusting PEEP via esophageal manometry to maintain positive transpulmonary pressures resulted in decreases in both elastance and in DPL. Several studies have suggested possible improved outcomes using higher PEEP strategies in patients with ARDS [7, 19–22]. Recruitment leading to increased size of the “baby lung” with subsequent improved compliance [23–25] might be the dominant reason for this finding. However, high PEEP alone is unlikely to be beneficial in all patients. Inappropriate PEEP may in fact cause hemodynamic compromise [26], increased dead space fraction [27] and direct barotrauma with lung over-distension and worsened compliance [27–29].
Our data illustrate that a more targeted approach utilizing esophageal pressure measurements to account for chest wall dynamics may better characterize a “best PEEP” for an individual patient. Although esophageal manometry represents mid-thorax pleural pressures, the net effect of this PEEP optimization appears to improve overall elastance and DP. Finding this “best PEEP” may optimize alveolar recruitment, increasing the size of the “baby lung” and reducing repetitive alveolar opening and closing (atelectrauma), while limiting over-distension and lung injury. Although mean PEEP increased in the intervention group, PEEP was not increased in all cases and the benefit from esophageal pressure monitoring appears to be more nuanced than simply attributing the improved DPL to the observed increase in PEEP.
Respiratory system versus transpulmonary monitoring
Amato et al. suggested that DPRS would be a reasonable surrogate for DPL in their analysis [13]; however, the results of our study may question this assessment. Although the majority of the respiratory system driving pressure was accounted for by the lungs, a significant portion (roughly 33 % on average) was secondary to the influence of the chest wall. Despite the LOWESS plots reflecting the expected linear relationship between DPRS and DPL, these plots illustrate the challenge to estimate the DPL for any given patient based upon the measured DPRS, with significant variability in chest wall elastance likely secondary to abdominal distension, obesity or chest wall edema which might contribute noise to the DPRS signal, not reflecting underlying lung properties. In theory, by excluding chest wall effects, DPL may be superior to DPRS as the more accurate marker of lung distending pressures, and utilizing esophageal manometry to estimate and remove the chest wall component may be superior to standard respiratory system measurements using airway pressures. Interestingly, we did not see a significant decrease in DPRS at 24 h in the intervention group despite the statistically significant decrease in DPL. While the current study lacks the statistical power to test the hypothesis that DPL is superior to DPRS, the observations in our study support further tests of this hypothesis. As DPRS appeared to be at least equal to DPL in terms of mortality correlation, it currently remains unclear if either measurement is superior.
Mortality prediction
With respect to the use of DP as an outcome predictor, Amato et al. proposed that scaling VT to body weight and “normalizing” to lung size was insufficient as functional lung size in ARDS is markedly decreased [13]. These authors hypothesized that this “baby lung” [24] is manifested as lower respiratory system compliance (CRS) and that “normalizing VT to CRS and using the ratio as an index of the “functional’ size of the lung” would be superior to VT alone and provide a better predictor of outcomes [13]. This “normalization” the authors refer to is the measured DPRS, which they found to be the strongest predictor of mortality in patients with ARDS. In our study, DPRS and DPL both decreased by 24 h in the 28 day survivor group, suggesting its possible use for prognostication. As elastance was similarly lower in survivors, it is unclear if DP is independently prognostic, and DPRS, ERS, DPL and EL could be further tested in a larger sample size. Ultimately it remains unclear from our data if DP might be useful for prognostication or if higher DP is simply another marker for poorly compliant lungs.
Driving pressure manipulation
It has also been suggested that manipulation of DP could be used for ventilator management at the bedside [13]. Theoretically, DP could be adjusted by changing the VT (low VT would similarly lower DP) and by adjusting the PEEP (to optimize compliance). The effects of PEEP adjustment on DP in our study were not seen at the 5 min time point. If there is a delayed response, titration of interventions designed to influence DP in real-time might be challenging. Given that we had data only at 5 min and 24 h, future studies will be needed to clarify the optimal time to determine these changes.
Limitations
There are several significant limitations to this study that will need to be addressed in future investigations. The small sample size and the unequal number of subjects in the survivor and non-survivor groups makes meaningful interpretation of the data challenging, as do the small (but equal) numbers when comparing by intervention. These small numbers do not allow for multivariate analysis to determine if DP might emerge as an independent predictor of mortality and limit our direct comparison of DPRS and DPL. Furthermore, the retrospective nature of this analysis weakens the interpretation as driving pressure was not a pre-specified endpoint in the initial study.
Conclusions
To our knowledge this is the first study to evaluate DPL via esophageal manometry as a tool to optimize ventilatory settings in ARDS patients. Although DPL appeared to be the superior to DPRS for monitoring changes in pulmonary mechanics, further investigation is needed to determine if DPL or DPRS is better for following mechanics and predicting mortality. These data lend support for future studies on interventions designed to improve driving pressure to determine if DPL or DPRS can be directly targeted at the bedside to improve outcomes.
References
Bernard GR, Artigas A (2016) The definition of ARDS revisited: 20 years later. Intensive Care Med 42:640–642. doi:10.1007/s00134-016-4281-z
Bernard GR, Artigas A, Brigham KL (1994) The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824. doi:10.1164/ajrccm.149.3.7509706
Wang CY, Calfee CS, Paul DW et al (2014) One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 40:388–396. doi:10.1007/s00134-013-3186-3
Villar J, Blanco J, Kacmarek RM (2016) Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care 22(1):1–6. doi:10.1097/MCC.0000000000000266
Anonymous (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med 342:1301–1308. doi:10.1056/NEJM200005043421801
Amato MB, Barbas CS, Medeiros DM et al (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354. doi:10.1056/NEJM199802053380602
Briel M, Meade M, Mercat A et al (2010) Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303:865–873. doi:10.1001/jama.2010.218
Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A (2006) A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 34:1311–1318. doi:10.1097/01.CCM.0000215598.84885.01
Gattinoni L, Quintel M (2016) Is mechanical ventilation a cure for ARDS? Intensive Care Med 42:916–917. doi:10.1007/s00134-016-4266-y
Dreyfuss D, Hubmayr R (2016) What the concept of VILI has taught us about ARDS management. Intensive Care Med 42:811–813. doi:10.1007/s00134-016-4287-6
Slutsky AS (1999) Lung injury caused by mechanical ventilation. Chest J 116:9S–15S
Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 369:2126–2136. doi:10.1056/NEJMra1208707
Amato MB, Meade MO, Slutsky AS et al (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372:747–755. doi:10.1056/NEJMsa1410639
Loring SH, Malhotra A (2015) Driving pressure and respiratory mechanics in ARDS. N Engl J Med 372:776–777. doi:10.1056/NEJMe1414218
Talmor D, Sarge T, Malhotra A et al (2008) Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 359:2095–2104. doi:10.1056/NEJMoa0708638
Talmor D, Sarge T, O’Donnell CR, Ritz R, Malhotra A, Lisbon A, Loring SH (2006) Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med 34:1389–1394. doi:10.1097/01.CCM.0000215515.49001.A2
Akoumianaki E, Maggiore SM, Valenza F et al (2014) The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 189:520–531. doi:10.1164/rccm.201312-2193CI
Beitler JR, Goligher EC, Schmidt M et al (2016) Personalized medicine for ARDS: the 2035 research agenda. Intensive Care Med 42:756–767. doi:10.1007/s00134-016-4331-6
Brower RG, Lanken PN, MacIntyre N et al (2004) Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351:327–336. doi:10.1056/NEJMoa032193
Grasso S, Fanelli V, Cafarelli A, Anaclerio R, Amabile M, Ancona G, Fiore T (2005) Effects of high versus low positive end-expiratory pressures in acute respiratory distress syndrome. Am J Respir Crit Care Med 171:1002–1008 (200407-940OC [pii])
Meade MO, Cook DJ, Guyatt GH et al (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299:637–645. doi:10.1001/jama.299.6.637
Mercat A, Richard JC, Vielle B et al (2008) Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299:646–655. doi:10.1001/jama.299.6.646
Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M (1987) Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis 136:730–736. doi:10.1164/ajrccm/136.3.730
Gattinoni L, Pesenti A (2005) The concept of “baby lung”. Intensive Care Med 31:776–784. doi:10.1007/s00134-005-2627-z
Gattinoni L, Marini JJ, Pesenti A, Quintel M, Mancebo J, Brochard L (2016) The “baby lung” became an adult. Intensive Care Med 42:663–673. doi:10.1007/s00134-015-4200-8
Luecke T, Pelosi P (2005) Clinical review: positive end-expiratory pressure and cardiac output. Crit Care 9:607–621 (cc3877 [pii])
Suter PM, Fairley B, Isenberg MD (1975) Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med 292:284–289. doi:10.1056/NEJM197502062920604
Gattinoni L, Caironi P, Cressoni M et al (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Eisner MD, Thompson BT, Schoenfeld D, Anzueto A, Matthay MA, Network Acute Respiratory Distress Syndrome (2002) Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. Am J Respir Crit Care Med 165:978–982. doi:10.1164/ajrccm.165.7.2109059
Acknowledgments
The authors acknowledge Robert Gerber for assistance in making the figures and Victor Novak for assistance with statistical analysis. There was no separate funding source for this study, however the original EPVent study was funded under Stephen Lorings RO1 Grant HL-52586.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Take-home message: Strategies titrating PEEP to target positive transpulmonary pressure in ARDS result in lower respiratory system and transpulmonary driving pressure secondary to improved elastance. Decreased respiratory system and transpulmonary driving pressures at 24 h were associated with improved 28 day morality, and changes in driving pressure with inventions were not seen immediately.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2016_4403_MOESM1_ESM.tif
Electronic Supplemental Material. a Relationship between DPRS and DPL at any time point. b Relationship between the change in DPRS (baseline to 5 min and baseline to 24 h) and change in DPL in individual subjects. Data were analyzed using locally weighted scatterplot smoothing (LOWESS) (TIFF 507 kb)
Rights and permissions
About this article
Cite this article
Baedorf Kassis, E., Loring, S.H. & Talmor, D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med 42, 1206–1213 (2016). https://doi.org/10.1007/s00134-016-4403-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4403-7