Abstract
Purpose
Requiring PaO2/FiO2 ratio (PF) to define ARDS may bias towards children with cardiovascular dysfunction and hypoxemia. We sought to evaluate (1) the Berlin definition of ARDS in children using PF; (2) the effect of substituting SpO2/FiO2(SF) ratio; (3) differences between patients with and without arterial blood gases; and (4) the ability of SpO2 and PaO2 indices to discriminate ICU mortality.
Methods
Single center retrospective review (3/2009–4/2013) of mechanically ventilated (MV) children. Initial values for PF, SF, oxygenation index (OI), and oxygen saturation index (OSI) after intubation and average values on day 1 of MV were analyzed against ICU mortality, subgrouped by Berlin severity categories.
Results
Of the 1,833 children included, 129 met Berlin PF ARDS criteria (33 % mortality); 312 met Berlin SF ARDS criteria (22 % mortality). Children with a PaO2 on day 1 of MV had higher mortality and severity of illness, were older, and had more vasoactive-inotropic infusions (p < 0.001). SF could be calculated for 1,201 children (AUC for ICU mortality 0.821), OSI for 1,034 (0.793), PF for 695 (0.706), and OI for 673 (0.739). Average SF on day 1 discriminated mortality better than PF (p = 0.003).
Conclusions
Berlin PF criteria for ARDS identified less than half of the children with ARDS, favoring those with cardiovascular dysfunction. SF or OSI discriminate ICU mortality as well as PF and OI, double the number of children available for risk stratification, and should be considered for severity of illness scores and included in a pediatric-specific definition of ARDS. Multicenter validation is required.
Similar content being viewed by others
Introduction
Arterial blood gas (ABG)-based measures of hypoxemia such as PaO2/FiO2 (PF) ratio have been used in ICU severity of illness scores [1–5], and as diagnostic criteria for acute respiratory distress syndrome (ARDS) [6, 7]. The Berlin definition (Berlin) has established new diagnostic criteria based exclusively on adult data and consensus of adult practitioners for ARDS [8], but has limited validation in children [9]. Berlin created three ARDS severity classes using PF with a minimum PEEP of 5 cmH2O. It requires a risk factor within 7 days of hypoxemia, bilateral infiltrates, and allows ARDS to co-exist with heart failure. The draft definition considered ancillary measures for severe ARDS (three quadrants of consolidation on chest x-ray, dead space, PEEP ≥10 cmH2O, and compliance) that were omitted from the final definition because they did not discriminate mortality.
When applying Berlin to pediatric ARDS patients, it is important to consider pediatric PEEP management, radiology, and measures of dead space or compliance. These may alter the ability of Berlin to risk stratify children with ARDS [10]. Perhaps most importantly, improvements in pulse oximetry have shifted practice patterns in pediatric intensive care to reduced use of arterial catheters; hence fewer children have a PF ratio to diagnose ARDS [11, 12]. Moreover, recent evidence that arterial catheters are an under-recognized source of infection [13] may continue to shift practice patterns away from routine use. Several studies have demonstrated that SpO2-based parameters such as SpO2/FiO2 ratio can be substituted for PF ratio when the SpO2 is ≤97 % [14–17].
There is good evidence that PF or oxygenation index (OI) [(mean airway pressure × FiO2/PaO2) × 100] discriminates mortality risk in children with ARDS [18–21], but few studies have examined whether SpO2-based metrics (SF or oxygenation saturation index (OSI) [(mean airway pressure × FiO2/SpO2) × 100]) discriminate mortality [22, 23] in children.
The objectives of this study are (1) to evaluate the performance of the Berlin definition for pediatric ARDS; (2) to determine how substituting SF for PF in the Berlin definition would alter ARDS incidence and mortality; (3) to examine the potential for selection bias introduced when requiring an ABG by examining differences between children with and without a PaO2 available on the first day of mechanical ventilation; and (4) to compare the ability of SpO2 vs. PaO2-based metrics of hypoxemia to discriminate mortality in a heterogeneous cohort of mechanically ventilated children (not just those with ARDS).
Materials and methods
We reviewed the electronic health records (EHR) of children admitted to the Children’s Hospital Los Angeles (CHLA) Pediatric Intensive Care Unit (PICU) from March 2009 to April 2013. Patients were eligible if intubated (endotracheal or tracheostomy) and mechanically ventilated (MV) within 7 days of PICU admission. Cyanotic congenital heart disease patients were excluded. IRB approval was obtained at CHLA (CCI-12-00140), in accordance with the 1964 Declaration of Helsinki and its later amendments.
Variable selection
We used a copy of the EHR (Cerner Corp®, Kansas City, MO), and a diagnostic and demographic database (Microsoft Access, Redmond, WA) maintained by physicians providing clinical care. Demographics, diagnoses, outcomes, ventilator settings, SpO2, ABGs, doses and times of vasoactive-inotropic medications, and 12-h admission pediatric risk of mortality (PRISM III) scores were extracted. Masimo pulse oximeters™ were used for all patients. There was no protocol regarding ABGs or the decision to place an arterial catheter. There has been a recent trend to rely on non-invasive parameters or capillary or venous blood gases, and only place arterial catheters for patients who need hemodynamic monitoring or have severe hypoxemia. Nurses and respiratory therapists primarily changed FiO2, with a guideline to wean FiO2 when >0.40 if SpO2 was >97 %.
Composite variable definitions
Data to calculate PF, SF, OI, and OSI were extracted for the first day of MV. SF and PF were calculated using the closest charted FiO2 ≤1 h from the SpO2 ≤97 % or PaO2. SpO2 >97 % were excluded. OI and OSI were calculated using the PF and SF values, incorporating the closest charted mean airway pressure (MAP) ≤6 h prior to SF or PF as previously described [18, 22]. Ventilator settings were charted every 6 h, or with changes. SpO2 was charted hourly, or with desaturation. We analyzed the first PF, SF, OI, and OSI after intubation, as well as average values over the first day of MV. The average values for SF and OSI were calculated using hourly average SpO2 values ≤97 %, preventing over-weighting times of instability, when there were several SpO2 values in each hour (e.g., SpO2 values charted between 12:00 and 13:00 were 97, 93, and 89 %. The hourly average for 12:00 is 93 %). Hourly average values ≤97 % were then averaged to derive the day 1 value. An hourly inotrope score was calculated for the first 3 days of ICU admission, using hourly average values of continuous dopamine, dobutamine, epinephrine, norepinephrine, vasopressin, and milrinone [24, 25].
Berlin definition ARDS criteria
Diagnostic risk factors for ARDS included pneumonia, sepsis, aspiration, pancreatitis, near drowning, trauma, and rheumatologic disease. Chest radiographs during the first 3 days of MV were reviewed for patients with a risk factor and either SF (≤264) or PF (≤300). Two independent reviewers examined x-rays and radiology reports for presence of bilateral infiltrates and cardiomegaly. Disagreements regarding bilateral infiltrates were adjudicated by a third reviewer. Patients with cardiomegaly had a review of their medical record to determine if hypoxemia was predominantly from heart failure. Ventilator settings were reviewed to ensure PEEP ≥5 cmH2O.
Outcome measures and analysis
The main objective was to evaluate the performance of Berlin to stratify ICU mortality risk in pediatric ARDS, using PF and SF criteria. Additional objectives were to examine differences between patients with and without an ABG, so descriptive statistics were stratified by the presence of an ABG on the first day of MV. Mortality and inotrope/vasopressor use were examined as a function of categories of SF, stratified by the presence of an ABG on the first day of MV. We used four categories of SF (>264, 221 to ≤264, 150 to ≤221, ≤150), corresponding to Berlin PF values (>300, 200 to ≤300, 100 to ≤200, ≤100) [16]. A final objective was to compare the abilities of PF, SF, OI, and OSI to discriminate mortality in MV children. Statistical analysis was performed using Statistica v.5.5 (StatSoft, Tulsa, OK) and Stata v.10 (StataCorp, College Station, TX). Continuous variables are described with median and interquartile range, analyzed with Wilcoxon rank-sum. Dichotomous variables are described as number and percent, analyzed using Yates-corrected chi squared. A multivariate logistic regression model was created to evaluate the independent association of SF ratio with mortality, controlling for potential confounding factors. Receiver operating characteristic (ROC) plots were created to examine the discrimination ability using area under the curve (AUC) [26] with 95 % confidence intervals. AUCs were compared using chi-squared tests.
Results
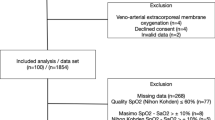
There were 4,803 ICU admissions during the study period with an overall mortality of 4.6 %. The study cohort consisted of 1,833 MV children without cyanotic congenital heart disease, with 9.9 % mortality.
Primary analysis: Berlin definition of ARDS
Stratification of mortality for patients who met Berlin ARDS criteria is summarized in Table 1. Overall, 129 children (7 % of MV children) met Berlin ARDS criteria, with 33 % mortality. There was similar mortality for children with mild (PF 200–300) or moderate (PF 100–200) ARDS using either initial PF after intubation, or day 1 average PF values, but higher mortality for children with severe ARDS (PF ≤100) (Table 1). The mild, moderate, and severe designation for ARDS severity using PF ratio had fair discrimination ability for mortality, which was similar for initial values after intubation [AUC 0.631 (95 % CI 0.54, 0.72)] and day 1 average values [AUC 0.665 (95 % CI 0.57, 0.76), p = 0.2] (Electronic Supplementary Material Fig. 1).
When SF criteria were substituted for PF in the Berlin definition, 312 children (17 % of MV children) met ARDS criteria, with 22 % mortality. There was similar mortality between those with mild (SF 221–264) or moderate (SF 150–221) ARDS, but higher mortality for those with severe ARDS (SF ≤150) when the initial SF after intubation was used (Table 1). However, there appeared to be clearer stepwise increases in mortality from mild to moderate to severe ARDS using day 1 average values for SF (Table 1). The mild, moderate, and severe designation for ARDS severity using SF ratio also had fair discrimination ability for mortality using initial values [AUC 0.632 (95 % CI 0.56, 0.69)], although it was significantly higher with day 1 average values [AUC 0.682 (95 % CI 0.62, 0.74), p = 0.002] (Electronic Supplementary Material Fig. 2).
Secondary analysis: selection bias with ABGs
To examine the selection bias if an ABG were required for risk stratification, we examined all 1,833 mechanically ventilated patients, stratified by presence of an ABG within 24 h of MV (Table 2). Those with ABGs had 16.1 % mortality as compared with 6.1 % mortality for those without ABGs (p < 0.0001). Patients with an ABG were older, ventilated longer, more likely to have cancer, and less likely to have chronic respiratory disease, a tracheostomy, or a home ventilator (all p < 0.02). Patients with ABGs on the first day of MV were treated with vasoactive-inotropic infusions sooner and had higher inotrope scores than those without ABGs (all p < 0.0002). Patients with ABGs had higher initial PRISM III severity of illness (p < 0.0001), with 23.8 % of patients with ABGs having predicted PRISM III risk of mortality ≥10 % as compared with 6.7 % for those without ABGs (p < 0.0001). There was a correlation between PRISM III and number of ABGs on day 1 of MV (r = 0.38, p < 0.0001).
Table 3 characterizes outcomes and interventions as a function of hypoxemia severity (measured by SF ratio). A total of 1,201 patients had SF available (those without SF ratio available had SpO2 >97 % precluding calculation). Hypoxemia severity correlated with the use of ABGs on day 1 of MV. However, 31 % of patients with severe hypoxemia (SF ≤150) did not have an ABG on the first day of MV, with 35 % mortality. There are stepwise increases in mortality as a function of SF, regardless of the presence of an ABG on the first day of MV (Table 3). Moreover, there is a stepwise increase in frequency of vasoactive-inotropic medication use as hypoxemia worsens (Table 3). Among patients with severe hypoxemia, 67 % of patients with an ABG on the first day of MV received vasoactive-inotropic medications within the first 24 h of PICU admission, whereas only 35 % of patients without an ABG received them (Table 3). SF was associated with mortality after controlling for presence of an ABG on day 1 of ventilation, vasoactive-inotropic medications within the first 24 h of PICU admission, age, and diagnoses of chronic respiratory disease or cancer (p < 0.001).
Secondary analysis: all MV patients
Four hypoxemia metrics (PF, SF, OI, and OSI) were calculated for all mechanically ventilated children (irrespective of ARDS diagnosis) when the necessary parameters were available. Availability of PF and SF, distribution, and mortality as a function of PF and SF severity ranges are summarized in Fig. 1. Allowing SF to be used when PF was not available doubled the number of patients available for risk stratification. For patients with only SF available, there is a clear step up in mortality for those with severe hypoxemia, with minimal difference between those with mild or moderate hypoxemia. For those with both PF and SF available, there are stepwise increases in mortality for SF ratio in this group, with a similar portion of patients in each severity group. For PF ratio, close to half of these children had day 1 average PF ratio >300, with similar mortality for those with PF ratios from 100 to 200 as 200 to 300. Nearly all patients who had PF but not SF available (i.e., SpO2 >97 %), had PF ratios >300 (Fig. 1).
Breakdown of population proportions and ICU mortality stratified by availability of PF ratio and SF ratio. The total population of mechanically ventilated children is 1,833, subgrouped along the x axis on the basis of availability of each metric (SF only, PF and SF, PF only, neither PF nor SF). The area of each box represents the proportion of the population which would fall into that severity group on the basis of the given metric. Proportions are based on day 1 average SF and PF. The percentage is mortality within each box. A total of 727 children had SF available, but no PF. While many of these patients had mild or minimal hypoxemia, those with severe hypoxemia (SF <150) had 38 % mortality. A total of 474 patients had both PF and SF available. There are stepwise increases in mortality for SF ratio in this group, with a similar portion of patients in each severity group. For PF ratio, close to half of these children had PF ratio >300, with similar mortality for those with PF ratios from 100–200 to 200–300 (note width of these boxes split in half for visualization). For those with PF ratio only (patients in whom SpO2 >97 % precluded SF calculation), 92 % of children had PF ratios >300. The remaining PF subgroups in this section had very few patients, likely affecting the mortality numbers (only five deaths total for PF ≤300). A total of 411 patients had neither metric available (SF not available because SpO2 was above 97 %)
When used as a continuous variable (rather than mild, moderate, or severe classifications), all four metrics (PF, SF, OI, and OSI) had “good” to “excellent” discrimination of ICU mortality, for both the initial value after intubation, and day 1 average values (Table 4) [27]. There was a median of two ABGs available for PaO2 metrics on day 1 of MV (range 1–17; IQR 1–4). For SpO2 metrics, there was a median of three hourly averaged SpO2 values ≤97 % available on day 1 of MV (range 1–22; IQR 1–7). In general, day 1 average values for all four metrics had higher discrimination of mortality than initial values after intubation (Table 4). SpO2 metrics increased the number of patients and had higher AUC than PaO2 metrics, particularly for the day 1 average values (Table 4). For direct comparison, we restricted the analysis to 444 children with all four metrics available (30 of the 474 from the analysis of PF and SF did not have MAP within 6 h for OI and OSI) and found similar discrimination ability for the initial values after intubation (Table 4). However, day 1 average SF had higher discrimination for mortality than PF (p = 0.0003). OSI and OI each tended to be better than PF at discriminating mortality, although not statistically significant (p = 0.06 and p = 0.07, respectively). All other comparisons were not different (p > 0.1). A total of 584 patients had only SpO2 metrics available, and mortality discrimination was similar (day 1, SF AUC 0.769; day 1, OSI AUC 0.752) to the group with both SpO2 and PaO2 available.
Discussion
We have demonstrated that the Berlin criteria identified 7 % of all MV children as having ARDS, with 33 % mortality. In contrast, using SF in the Berlin definition identified 17 % of MV children as having ARDS, with 22 % mortality. SpO2 metrics of hypoxemia (SF and OSI) could double the number of MV children available for risk stratification, and PaO2 metrics are available only for about half of children with moderate hypoxemia. Although 70 % of those with severe hypoxemia have an ABG, the remaining 30 % of patients with severe hypoxemia but without an ABG still have 35 % mortality. ABGs are likely a surrogate for cardiovascular dysfunction or severity of illness, as their use is associated with vasoactive-inotropic medications, higher PRISM scores, and higher mortality in pediatric ICUs. Finally, when looking at the performance of hypoxemia metrics of disease severity, the first PF, OI, SF, and OSI after intubation discriminate mortality similarly, whereas day 1 average values for SF may outperform PF.
Limiting diagnosis and risk stratification for ARDS to children who have only PF or OI available is capturing a combination of the hypoxemia and cardiovascular risk, rather than pulmonary organ dysfunction. However, before simply accepting an SF modified Berlin definition of ARDS for use in children, we should consider creating a pediatric-specific definition of ARDS involving important pediatric practice patterns, co-morbidities, and other markers of disease severity [28].
A major advantage of SpO2 metrics is the avoidance of selection bias regarding the decision to obtain an ABG or place an arterial catheter. In our PICU, it appears that children with ABGs are older, have different co-morbidities, have higher initial severity illness, and are more likely to receive vasoactive-inotropic infusions. Although mortality rates are higher for those with ABGs, SF and OSI discriminate mortality, regardless of the presence of an ABG. This is seen by stepwise increases in mortality as SF worsens, and similar AUCs for the ROC plots between the groups with and without ABGs. Moreover, SF remains associated with mortality after controlling for ABG use, inotrope-vasopressors, age, and diagnoses.
Interestingly, we found that the average SF on the first day of MV better discriminates mortality as compared with PF. There were similar trends for OSI and OI, although not statistically significant (p = 0.06 and 0.07, respectively). There are likely several reasons for this higher discrimination. First, because SpO2 is available routinely, one can calculate SF and OSI on more patients, with a larger spectrum of disease severity. Second, SpO2 may be a better marker of intrapulmonary shunt [29]. Third, SF and OSI have more data points to average than PF or OI, which may better capture the composite risk for the patient during day 1 of ventilation. This may be the reason why there was more equal distribution of patients and clearer stepwise increases in mortality when using day 1 average SF as compared with day 1 average PF for the 474 patients who had both metrics available (Fig. 1). We did not require simultaneous SF and PF, which may explain why patients do not always fall in the same severity buckets (i.e., severe hypoxemia via SF but moderate via PF). However, there were only a median of three hourly SpO2 values ≤97 % to average on the first day of MV, likely because providers do not wean FiO2 to keep SpO2 ≤97 %, as needed for SF and OSI [14, 16, 30]. Finally, when comparing initial values of SF or PF to day 1 average values for ARDS patients, there is a tendency for patients to move from severe to moderate ARDS, which may imply the day 1 value better reflects interventions such as lung recruitment. Day 1 mild, moderate, and severe ARDS designation also appears to better discriminate mortality than the initial value after intubation, particularly when using SF criteria.
One can debate whether a day 1 average value for any of these parameters should be used, as it may reflect quality of ICU interventions, not disease severity [7]. However, such combination metrics may be important for trials that do not enroll patients immediately after intubation, or when considering higher risk therapies for which duration of lung disease severity may be relevant. Future studies could be designed to require a minimum of two SF or OSI values, separated by at least an hour. Regardless, initial values of SF or OSI after intubation appear to discriminate mortality as well as the initial values for PF or OI [31–33].
It is logical to use SpO2 metrics for epidemiologic studies and severity of illness scores when a PaO2 is unavailable to increase the number of eligible patients and represent the spectrum of disease severity [34–37]. However, SpO2 metrics may be important to avoid selection bias of sicker patients with more cardiovascular dysfunction in interventional trials [38]. This may be particularly important when the intervention has potential for cardiopulmonary interactions (e.g., PEEP or high frequency oscillatory ventilation).
There are several limitations to our study, mostly related to the retrospective, single center design. We cannot have precise alignment of SpO2 or PaO2 with FiO2 and MAP for calculation of PF, SF, OI, and OSI. By requiring SpO2 to be within 1 h of FiO2 we have hoped to minimize this uncertainty (and still have demonstrated a positive association). We anticipate that this relationship may be stronger with precise alignment of FiO2 and SpO2 (or PaO2). We did not have an active protocol to wean FiO2 to maintain SpO2 ≤97 %. For this reason we had to eliminate many SpO2 values from the analysis. Third, we were limited to data that was charted, which may not reflect minute-to minute changes from bedside monitors. However, the EHR is the most frequent data source for severity of illness scores and eligibility for clinical trials, so our data likely represent real-life application. Fourth, we attempted standardized assessment with consensus opinion of bilateral infiltrates on CXR, but there is inherently high variability in this interpretation [39]. Fifth, SpO2 may be less reliable in circumstances of hemodynamic instability and poor perfusion, necessitating PaO2 measurements. Finally, these are data from a single institution, and are subject to local bias and practice patterns, particularly with regards to decisions to place arterial catheters. These findings need to be confirmed prospectively with multi-institutional data.
Conclusions
Children with severe ARDS using Berlin PF criteria have high mortality (45–53 %), with minimal difference in mortality between the mild and moderate ARDS groups. Substituting equivalent SF values for Berlin PF criteria creates slightly better separation in mortality risk between mild and moderate ARDS, although the severe group still has twice the mortality as the moderate group. SpO2 metrics (SF and OSI) discriminate mortality as well as PF and OI, increase the number of children eligible for the diagnosis of ARDS, and prevent limiting the diagnosis to those with hypoxemia and cardiovascular dysfunction. Although our study was performed in children, these findings may generalize to adults, and warrant investigation.
References
Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR, SAPS 3 Investigators (2005) SAPS 3–from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission.. Intensive Care Med 31:1345–1355; [Erratum appears in Intensive Care Med. 2006 May; 32(5):796]
Zimmerman JE, Kramer AA, McNair DS, Malila FM (2006) Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med 34:1297–1310
Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study Group (2003) PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 29:278–285
Pollack MM, Patel KM, Ruttimann UE (1996) PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 24:743–752
Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F, Groupe Francophone de Reanimation et d’Urgences Pédiatriques (2013) PELOD-2: an update of the pediatric logistic organ dysfunction score. Crit Care Med 41:1761–1773
Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, Rhodes A, Slutsky AS, Vincent J-L, Rubenfeld GD, Thompson BT, Ranieri VM (2012) The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 38:1573–1582
Villar J, Perez-Mendez L, Blanco J, Anon JM, Blanch L, Belda J, Santos-Bouza A, Fernandez RL, Kacmarek RM, Spanish Initiative for Epidemiology Stratification, and Therapies for ARDS (SIESTA) Network (2013) A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting—a prospective, multicenter validation study. Intensive Care Med 39:583–592
Hernu R, Wallet F, Thiolliere F, Martin O, Richard JC, Schmitt Z, Wallon G, Delannoy B, Rimmele T, Demaret C, Magnin C, Vallin H, Lepape A, Baboi L, Argaud L, Piriou V, Allaouchiche B, Aubrun F, Bastien O, Lehot JJ, Ayzac L, Guerin C (2013) An attempt to validate the modification of the American–European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Med 39:2161–2170
De Luca D, Piastra M, Chidini G, Tissieres P, Calderini E, Essouri S, Medina Villanueva A, Vivanco Allende A, Pons-Odena M, Perez-Baena L, Hermon M, Tridente A, Conti G, Antonelli M, Kneyber M, Respiratory Section of the European Society for Pediatric Neonatal Intensive Care (2013) The use of the Berlin definition for acute respiratory distress syndrome during infancy and early childhood: multicenter evaluation and expert consensus. Intensive Care Med 39:2083–2091
Khemani RG, Wilson DF, Esteban A, Ferguson ND (2013) Evaluating the Berlin definition in pediatric ARDS. Intensive Care Med 39:2213–2216
Khemani RG, Markovitz BP, Curley MA (2009) Characteristics of children intubated and mechanically ventilated in 16 PICUs. Chest 136:765–771
Santschi M, Jouvet P, Leclerc F, Gauvin F, Newth CJ, Carroll C, Flori H, Tasker RC, Rimensberger P, Randolph A, PALIVE Investigators, Pediatric Acute Lung Injury and Sepsis Investigators Network (PALISI), European Society of Pediatric and Neonatal Intensive Care (ESPNIC) (2010) Acute lung injury in children:therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med 11:681–689
O’Horo JC, Maki DG, Krupp AE, Safdar N (2014) Arterial catheters as a source of bloodstream infection: a systematic review and meta-analysis. Crit Care Med 42:1334–1339
Thomas N, Shaffer ML, Willson D, Shih M, Curley M (2010) Defining acute lung disease in children with the oxygen saturation index. Pediatr Crit Care Med 11:12–17
Khemani RG, Patel NR, Bart RD, Newth CJ (2009) Comparison of the pulse oximetric saturation/fraction of inspired oxygen ratio and the PaO2/fraction of inspired oxygen ratio in children. Chest 135:662–668
Khemani RG, Thomas NJ, Venkatachalam V, Scimeme JP, Berutti T, Schneider JB, Ross PA, Willson DF, Hall MW, Newth CJ, Pediatric Acute Lung I, Sepsis Network I (2012) Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med 40:1309–1316
Mayordomo-Colunga J, Pons M, Lopez Y, Jose Solana M, Rey C, Martinez-Camblor P, Rodriguez-Nunez A, Lopez-Herce J, Medina A, Abadesso C, Angeles Garcia-Teresa M, Gaboli M, Garcia-Lopez M, Gonzalez-Sanchez M, Madurga-Revilla P, Gonzalez-Calvar A, Onate E (2013) Predicting non-invasive ventilation failure in children from the SpO2/FiO2 (SF) ratio. Intensive Care Med 39:1095–1103
Khemani RG, Conti D, Alonzo TA, Bart RD, Newth CJ (2009) Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med 35:1428–1437
Flori HR, Glidden DV, Rutherford GW, Matthay MA (2005) Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 171:995–1001
Trachsel D, McCrindle BW, Nakagawa S, Bohn D (2005) Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med 172:206–211
Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, Wilkins B, Paediatric Study Group, Australian and New Zealand Intensive Care Society (2007) Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med 8:317–323
Ghuman AK, Newth CJ, Khemani RG (2012) The association between the end tidal alveolar dead space fraction and mortality in pediatric acute hypoxemic respiratory failure. Pediatr Crit Care Med 13:11–15
Leteurtre S, Dupre M, Dorkenoo A, Lampin ME, Leclerc F (2012) Assessment of the Pediatric Index of Mortality 2 with the PaO2/FiO2 ratio derived from the SpO2/FiO2 ratio: a prospective pilot study in a French pediatric intensive care unit. Pediatr Crit Care Med 12:e184–186; [Erratum appears in Pediatr Crit Care Med. 2012 Jan; 13(1):127]
Khemani RG, Bart RD, Alonzo TA, Hatzakis G, Hallam D, Newth CJL (2009) Disseminated intravascular coagulation score is associated with mortality for children with shock. Intensive Care Med 35:327–333
Rosenzweig EB, Starc TJ, Chen JM, Cullinane S, Timchak DM, Gersony WM, Landry DW, Galantowicz ME (1999) Intravenous arginine-vasopressin in children with vasodilatory shock after cardiac surgery. Circulation 100:182–186
David M, Karmrodt J, Bletz C, David S, Herweling A, Kauczor HU, Markstaller K (2005) Analysis of atelectasis, ventilated, and hyperinflated lung during mechanical ventilation by dynamic CT. Chest 128:3757–3770
Hosmer D, Lemeshow S (2000) Applied logistic regression. Wiley, New York
Thomas NJ, Jouvet P, Willson D (2013) Acute lung injury in children—kids really aren’t just “little adults”. Pediatr Crit Care Med 14:429–432
Dean JM, Wetzel RC, Rogers MC (1985) Arterial blood gas derived variables as estimates of intrapulmonary shunt in critically ill children. Crit Care Med 13:1029–1033
Khemani RG, Sward K, Morris A, Dean JM, Newth CJ, Collaborative Pediatric Critical Care Research Network (CPCCRN) (2011) Variability in usual care mechanical ventilation for pediatric acute lung injury: the potential benefit of a lung protective computer protocol. Intensive Care Med 37:1840–1848
Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA, Pediatric Acute Lung Injury Sepsis Investigators Network I (2005) Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA 293:470–476
Curley MA, Hibberd PL, Fineman LD, Wypij D, Shih MC, Thompson JE, Grant MJ, Barr FE, Cvijanovich NZ, Sorce L, Luckett PM, Matthay MA, Arnold JH (2005) Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA 294:229–237
Willson DF, Thomas NJ, Tamburro R, Truemper E, Truwit J, Conaway M, Traul C, Egan EE, Pediatric Acute Lung Injury Sepsis Investigators Network (2013) Pediatric calfactant in acute respiratory distress syndrome trial. Pediatr Crit Care Med 14:657–665
Piastra M, De Luca D, Marzano L, Stival E, Genovese O, Pietrini D, Conti G (2011) The number of failing organs predicts non-invasive ventilation failure in children with ALI/ARDS. Intensive Care Med 37:1510–1516
Kneyber MCJ, Brouwers AGA, Caris JA, Chedamni S, Plötz FB (2008) Acute respiratory distress syndrome: is it underrecognized in the pediatric intensive care unit? Intensive Care Med 34:751–754
Yu W-L, Lu Z-J, Wang Y, Shi L-P, Kuang F-W, Qian S-Y, Zeng Q-Y, Xie M-H, Zhang G-Y, Zhuang D-Y, Fan X-M, Sun B, Failure CSGoPR (2008) The epidemiology of acute respiratory distress syndrome in pediatric intensive care units in China. Intensive Care Med 35:136–143
Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD (2009) Incidence and outcomes of pediatric acute lung injury. Pediatrics 124:87–95
Tonelli AR, Zein J, Adams J, Ioannidis JP (2014) Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intensive Care Med 40:769–787
Angoulvant F, Llor J, Alberti C, Kheniche A, Zaccaria I, Garel C, Dauger S (2008) Inter-observer variability in chest radiograph reading for diagnosing acute lung injury in children. Pediatr Pulmonol 43:987–991
Acknowledgments
No specific funding was used for this manuscript. We would like to thank Paul Vee and Beverly Andrews for their assistance with data management, and Ricky Nguyen for his assistance with graphics.
Conflicts of interest
No conflicts of interest exist for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: This study has demonstrated that using Berlin PF criteria for pediatric ARDS would identify less than half of the children who likely have ARDS, resulting in a very high mortality group because it favors those with both hypoxemia and cardiovascular dysfunction. Using SpO2-based metrics such as SF or OSI would double the number of children available to be diagnosed with ARDS and represent a broader spectrum of patients with hypoxemia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
134_2014_3486_MOESM1_ESM.tif
Supplementary material 1 (TIFF 1821 kb) Fig. 1: ROC plots using mild, moderate, severe categorization for ARDS severity using the 129 patients who met ARDS criteria using PF ratio. Outcome is ICU mortality. Day 1 average values resulted in slightly higher AUC than initial values after intubation, although this was not statistically significant (p = 0.22).
134_2014_3486_MOESM2_ESM.tif
Supplementary material 2 (TIFF 1821 kb) Fig. 2: ROC plots using mild, moderate, severe categorization for ARDS severity using the 312 patients who met ARDS criteria using SF ratio. Outcome is ICU mortality. Day 1 average values resulted in significantly higher AUC than initial values after intubation (p = 0.002).
Rights and permissions
About this article
Cite this article
Khemani, R.G., Rubin, S., Belani, S. et al. Pulse oximetry vs. PaO2 metrics in mechanically ventilated children: Berlin definition of ARDS and mortality risk. Intensive Care Med 41, 94–102 (2015). https://doi.org/10.1007/s00134-014-3486-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3486-2