Abstract
In critically ill patients monitored with an arterial catheter, the arterial pressure signal provides two types of information that may help the clinician to interpret haemodynamic status better: the mean values of systolic, diastolic, mean and pulse pressures; and the magnitude of the respiratory variation in arterial pressure in patients undergoing mechanical ventilation. In this review we briefly discuss the physiological mechanisms responsible for arterial pressure generation, with special focus on resistance, compliance and pulse wave amplification phenomena. We also emphasize the utility of taking into consideration the overall arterial pressure set (systolic, diastolic, mean and pulse pressures) in order to define haemodynamic status better. Finally, we review recent studies showing that quantification of respiratory variation in pulse and systolic arterial pressures can allow one to identify the mechanically ventilated patients who may benefit from volume resuscitation.
Similar content being viewed by others
Introduction
Most physicians currently use the maximal (systolic) and minimal (diastolic) arterial pressure to assess cardiovascular status because these two pressures are easily measurable using a sphygmomanometer. For example, hypertension is defined as a systolic pressure of 140 mmHg or above or a diastolic pressure of 90 mmHg or above [1]. Recent studies have increased clinical interest in also analyzing other pressures, especially pulse pressure (PP) and mean arterial pressure (MAP). In this article we focus on the interpretation of arterial pressure wave in critically ill patients monitored with an arterial catheter. The arterial pressure signal can provide two types of information that may help the clinician to interpret haemodynamic status better: the mean values of systolic arterial pressure (SAP) and diastolic arterial pressure (DAP), and MAP and PP; and the magnitude of the respiratory variation in arterial pressure.
Physiological background
Aortic pressure
The arterial pressure wave can be described in terms of its steady and pulsatile components [2, 3]. The steady component is the MAP, which is considered constant from aorta to peripheral large arteries. The arterial pressure signal oscillates around this mean value in a complex manner. A simplified analysis relies on measurement of maximal and minimal arterial pressures values (i.e. SAP and DAP), which allows calculation of arterial PP (PP = SAP - DAP). Although DAP is roughly constant from aorta to periphery, SAP and therefore PP increase from aorta to periphery in young, healthy individuals. The degree of this so-called pulse wave amplification is about 15 mmHg on average, and it may vary depending on physiological (e.g. sex, age, heart rate, body height) or pathological (e.g. changes in vasomotor tone and arterial stiffness) conditions [3]. Thus, unlike MAP and DAP, peripheral SAP and PP are not necessarily reliable estimates of central pressure values.
The key equation governing human haemodynamics refers to the steady components of pressure and flow. The driving pressure of the systemic circulation is MAP minus the mean systemic pressure. The mean systemic pressure is the theoretical pressure value that would be observed in the overall circulatory system under zero flow conditions. As derived from Ohm's law, the driving pressure is the product of cardiac output (CO) and systemic vascular resistance (SVR) [4]. Given that mean systemic pressure cannot routinely be measured, mean right atrial pressure (mRAP) is currently taken as a surrogate, such that MAP can be expressed as follows:
MAP = (heart rate × SV × SVR) + mRAP (1)
Where SV is the stroke volume. Three important points must be stressed. First, SVR is not a measured parameter but is calculated from the measured values of MAP, CO and mRAP. Second, despite clear limitations in Poiseuille's Law when it is applied to the human circulation, it is generally believed that SVR is inversely proportional to the fourth power of the functional radius of the systemic network (mainly that of the distal resistive arteries). Finally, for a given MAP, SVR depends only on the value of CO, regardless of the way in which CO is generated (e.g. low SV/high heart rate or high SV/low heart rate).
It is often assumed that mRAP is small enough that it may be neglected in comparison with MAP, thus allowing the calculation of the MAP/CO ratio (total peripheral resistance). However, this statement is commonly not valid in hypotensive patients, especially those with right heart failure.
Systemic arteries are not just resistive conduits that distribute CO to peripheral organs. Rather, systemic arteries (especially the proximal aorta) are elastic structures that dampen the discontinuous ventricular ejection by storing a fraction of the SV in systole and restoring it in diastole, thus allowing continuous blood flow at the organ level. Therefore, the human circulation can be reasonably described by using the simplified Windkessel model, in which a capacitive element (total arterial compliance) is added to SVR. In this model, the compliance value has been accurately estimated as follows [5]:
Compliance = SV/aortic PP (2)
In fact, the arterial pressure wave has a complex transmission across the branching and tapering of the arterial tree, and travels with a high pulse wave velocity (about 8–10 m/s). As a result, wave reflections occur at aortic level and take place in early diastole in healthy individuals, with a beneficial boosting effect on coronary filling. This latter effect is lost where arterial stiffness is increased (e.g. in aged individuals), and wave reflection then occurs in late systole, thus increasing the afterload of the still-ejecting left ventricle. Analysis of the timing and extent of wave reflection adds valuable information regarding the pulsatile load imposed on the left ventricle [6, 7].
Peripheral arterial pressure
From a local point of view, peripheral arterial pressure is a function of both the distending blood volume and the compliance of the artery under study. By using an integrated dynamic description, peripheral arterial pressure is mainly determined by the pressure at the aortic root level and the characteristics of arterial pressure wave transmission and reflection. SV, arterial stiffness (1/compliance), heart rate, MAP and the distance from aorta to peripheral artery influence the pulsatile arterial pressure at the peripheral site.
There is a tight mathematical relationship between SAP, DAP, PP and MAP. Indeed, in the general population [1–3, 8] as well as in critically ill patients [9], the true, time averaged MAP can be accurately calculated according to the following classic empirical formula:
MAP = DAP + 1/3(SAP - DAP) (3)
This formula can be rewritten as follows:
MAP = (2/3 × DAP) + (1/3 × SAP) (4)
In other words, this rule of thumb implies that DAP contributes more to MAP, by a factor of two, than does SAP.
From a theoretical point of view, arterial compliance cannot be quantified by means of a single number because compliance decreases when MAP increases. Otherwise stated, the distending volume/distending pressure relationship has a curvilinear shape and the systemic vessels are stiffer (i.e. less easily distensible) at higher levels of mean distending pressure [10]. However, it is believed that this phenomenon plays only a moderate role over the physiological range of MAP observed in clinical practice.
Clinical correlates
Background
In the intensive care unit, arterial pressure can be monitored with either invasive or noninvasive techniques. It is beyond the scope of this review to detail the technical aspects of such monitoring. In brief, the oscillometric, noninvasive devices measure MAP (point of maximal oscillation), whereas estimation of SAP and DAP is obtained from various algorithms, depending on the device used. The method may be inaccurate in patients with marked changes in peripheral vascular tone, either primary or secondary to compensatory mechanisms or to the use of vasoactive agents. Therefore, patients with circulatory shock are often equipped with an intra-arterial catheter to obtain more accurate arterial pressure measurements. Furthermore, this is the only way to visualize the entire arterial pressure curve, and this also allows easy blood withdrawal for repeated biochemical analyses.
Although the aortic pressure curve shape per se contains valuable haemodynamic information, precise analysis of the shape of the peripheral arterial pressure curve cannot be recommended for assessment of haemodynamic status at the bedside. Indeed, there are major differences between peripheral and aortic pressure waves because of complex propagation and reflection wave phenomena. Furthermore, fluid-filled catheter and transducer characteristics lead to unavoidable distortion of the signal. Sources of measurement error have been widely discussed and may relate to various factors, including the transducer–tubing–catheter subsystem [11, 12]. Testing the system and avoiding underdamping, overdamping, zeroing and calibration errors are prerequisites for optimal analysis of arterial pressure values and arterial pressure waveform. Frequency response problems are especially evident in pressure measurements. As with any complex signal waveform, the arterial pressure waveform can be constructed by combining sine waves at different frequencies, amplitudes, and phases, as discussed previously [11, 12]. Frequency response is a measure of an instrument's ability to measure an oscillating signal accurately. A system is said to be damped when some of the signal frequencies are attenuated, and one must seek optimal damping.
In the remaining part of this review we focus on the informative value of the four pressures routinely measured (MAP, SAP, DAP and PP) and the clinical significance of respiratory changes in arterial pressure in patients with circulatory shock.
Informative value of mean arterial pressure
Both baseline MAP and changes in MAP must be explained by the combined influences of heart rate, SV, SVR and mRAP (Eqn 1). Autoregulation of the MAP is a key feature of the cardiovascular system. Acute decreases in MAP are counteracted by the sympathetically mediated tachycardia, increases in SV (mediated via positive inotropic effect and veno-constriction) and arterial systemic vasoconstriction. In critically ill patients, especially those with sepsis or who are receiving sedative drugs, these compensatory mechanisms can be either impaired or overwhelmed.
The constancy of MAP in large arteries explains why MAP is considered the driving pressure for perfusion of most vital organs [10]. As a result, when MAP falls below the lower limit of autoregulation, regional blood flow becomes linearly dependent on MAP. In some pathological settings, MAP overestimates the true perfusion pressure because of marked increases in extravascular pressure at the outflow level in specific vascular areas (intracranial hypertension, abdominal compartment syndrome) or because of marked increases in systemic venous pressure (right heart failure).
There is no universally accepted MAP threshold that provides assurance that blood flow is independent of arterial pressure in most vital organs. Indeed, the critical level of MAP probably differs among organs and depends on numerous factors, including age, previous history of hypertension, neuro-vegetative state and vasoactive therapy. Thus, there is no single 'magic value' for therapeutic MAP goals in shock states. However, in septic shock current resuscitation guidelines [13, 14] recommend that an MAP of 65 mmHg or greater be achieved and maintained, in order to avoid additional organ hypoperfusion. On the other hand, increasing MAP to 85 mmHg does not result in improved tissue oxygenation and regional perfusion [15, 16]. Finally, optimal MAP goals may be significantly greater in certain, subgroups including aged or previously hypertensive individuals.
Informative value of pulse pressure
Although it remains to be demonstrated, it is widely accepted that peripheral PP at rest depends mainly on SV and arterial stiffness (1/compliance) [3, 8]. In this regard, in older individuals increased arterial stiffness leads to increased PP, and this results in systolic hypertension associated with decreased DAP. On the other hand, in patients with cardiogenic or hypovolaemic shock, decreased SV results in a lower PP. The paradoxical finding of a low PP in the elderly and in patients with hypertension or atherosclerosis strongly suggests that SV is markedly low (unpublished observation) because arterial stiffness is expected to be increased in these patients.
It is likely that the monitoring of short-term PP changes in critically ill patients may provide valuable, indirect information on concomitant SV changes. In this regard, increases in PP induced by passive leg raising are linearly related to concomitant SV changes in mechanically ventilated patients [17].
Informative value of systolic and diastolic arterial pressures
The various patterns of arterial pulse observed with ageing [18] and in chronic hypertensive states [19] may help us to understand the haemodynamic correlates of SAP and DAP. Increases in the tone of distal muscular arteries is the landmark of systolic/diastolic hypertension, with increased MAP and essentially unchanged PP because of congruent increases in SAP and DAP. This pattern is typically observed in the early stages of essential hypertension in young or middle-aged individuals. Alternatively, increased stiffness of proximal elastic arteries is the landmark of systolic hypertension, with increased PP, increased SAP and decreased DAP. Increased SAP contributes to left ventricular pressure overload and increased oxygen demand, whereas decreased DAP can potentially compromise coronary perfusion and oxygen supply. This pattern is typically observed at the late stages of essential hypertension in elderly individuals [19]. Arterial pulse patterns that combine the two typical patterns described above can also be observed. Finally, it must be noted that isolated increases in SV may help to explain the isolated systolic hypertension observed in young patients [20].
In clinical practice, differences in mean DAP values are believed to reflect mainly changes in vascular tone, with lower DAP corresponding to decreased vascular tone. As discussed above, and for a given MAP, increased arterial stiffness also tends to be associated with lower DAP (and higher SAP as well). According to the classic MAP empirical formula, and for a given MAP, an increase in arterial stiffness increases SAP twofold more than it decreases DAP. Finally, from a beat-to-beat point of view, prolonged diastolic time is associated with lower DAP and shorter diastolic time with higher DAP.
Clinical scenario
The knowledge of these four arterial pressure values, namely SAD, DAP, MAP and PP, allows rational analysis of haemodynamic status, especially in patients with circulatory shock. This can be summarized in the following clinical scenario, in which two elderly patients admitted to the emergency room for circulatory shock and exhibiting the same 80 mmHg SAP may require different management because of markedly different haemodynamic profiles.
Let us define patient A as having 35 mmHg DAP and patient B as having 60 mmHg DAP. Despite similar SAP, the two patients differ markedly in terms of the steady component of arterial pressure (MAP = 50 mmHg in patient A; MAP = 67 mmHg in patient B) and the pulsatile component of arterial pressure (PP = 55 mmHg in patient A; PP = 20 mmHg in patient B). It is intuitive that patient A requires urgent and aggressive therapy to increase the markedly low MAP (in order to prevent vital organ hypoperfusion) and DAP (to prevent myocardial ischaemia). It is likely that vascular tone is reduced in patient A and that vasopressor therapy is urgently required. In patient B, the salient feature is the markedly decreased PP. Given the advanced age of the patient, the unexpected finding of a low PP strongly suggests that the SV is dramatically reduced. This implies that fluid challenge and/or inotropic support may be required.
Respiratory variations in arterial pressure
Background
In patients receiving mechanical ventilation, the magnitude of the ventilatory cyclic changes in arterial pressure has been proposed as a marker of the degree of hypovolaemia [21] and of volume responsiveness [22–24]. The rationale underpinning the use of such a marker is based on the hypothesis that the degree of heart–lung interaction is mainly related to the presence of cardiac preload reserve. It is beyond the scope of this review to describe the precise mechanisms involved, which are detailed in previous reviews [23, 24]. Briefly, mechanical ventilation should result in significant changes in left ventricular SV only if both ventricles have some preload reserve [23, 24]. Because a significant haemodynamic response to fluid should occur only under biventricular preload dependent conditions, it has been logically postulated that the magnitude of cyclic changes in SV should correlate with the degree of fluid responsiveness [23, 24].
Respiratory changes in pulse pressure
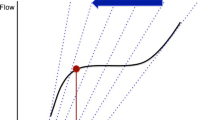
The arterial PP is directly proportional to left ventricular SV and inversely related to compliance of the arterial system. Assuming that arterial compliance does not change during a mechanical breath, respiratory changes in left ventricular SV should be reflected by respiratory changes in peripheral PP (ΔPP). Accordingly, the magnitude of ΔPP has been proposed as a marker of the degree of haemodynamic response to fluid loading [25]. The ΔPP is calculated as the difference between the maximal (PPmax) and the minimal (PPmin) values of PP over a single respiratory cycle, divided by the average of the two values, and expressed as a percentage (Fig. 1): ΔPP (%) = (PPmax - PPmin)/([PPmax + PPmin]/2) × 100.
Respiratory changes in arterial pressure in a mechanically ventilated patient. The pulse pressure (PP; systolic minus diastolic pressure) is minimal (PPmin) three heart beats after its maximal value (PPmax). The respiratory changes in pulse pressure (ΔPP) can be calculated as the difference between PPmax and PPmin, divided by the mean of the two values, and expressed as a percentage: ΔPP (%) = 100 × (PPmax - PPmin)/([PPmax + PPmin]/2). In this case, the high value of ΔPP (30%) suggests that the patient would be potentially responsive to volume resuscitation.
In septic shock patients receiving controlled ventilation, a ΔPP threshold value of 13% allowed discrimination between responders (ΔPP ≥ 13%) and nonresponders (ΔPP <13%) to volume resuscitation with high positive and negative predictive values [25]. Moreover, the higher the ΔPP was at baseline, the greater the increase in CO in response to fluid infusion [25]. Furthermore, the decrease in ΔPP associated with fluid infusion was correlated with the increase in CO. Thus, ΔPP may be helpful not only in predicting but also in monitoring the haemodynamic effects of volume expansion. It must be noted that neither baseline mRAP nor baseline pulmonary artery occlusion pressure predicted the haemodynamic response to volume infusion in that study [25], which confirms the poor reliability of filling pressures in detecting fluid responsiveness [26]. Similar results were reported in patients mechanically ventilated for acute respiratory distress syndrome [27], in cardiac surgery patients [28, 29] and in a general population of critically ill patients [30].
New real-time haemodynamic monitoring devices automatically calculate and continuously display ΔPP values.
Respiratory changes in systolic arterial pressure and its Δdown component
Analysis of respiratory changes in SAP (ΔSAP) has also been proposed as a marker of fluid responsiveness [21, 22]. However, ΔSAP depends not only on changes in SV but also on the cyclic direct effects of intrathoracic pressure on the thoracic aorta wall [31]. Therefore, significant ΔSAP can theoretically be observed in nonresponding patients. Accordingly, ΔSAP have been shown to be slightly less valuable than ΔPP in detecting volume responsiveness [25, 28, 29]. ΔSAP is useful in settings where ΔPP monitoring is not available, given its superiority over static indices of preload in assessing preload reserve [25, 29].
It has been proposed that an end-expiratory pause should be performed to separate the inspiratory increase in SAP (Δup, not always due to an increase in SV) and the expiratory decrease in SAP (Δdown). The Δdown component reflects the expiratory decrease in left ventricular SV [21]. In patients with septic shock, a baseline Δdown threshold value of 5 mmHg was demonstrated to distinguish responders from nonresponders to fluid administration better than static markers of cardiac preload [22]. The Δup component reflects the inspiratory increase in systolic pressure, which may result from numerous factors: increases in left ventricular SV related to the increase in LV preload (squeezing of blood out of alveolar vessels); increases in left ventricular SV related to a decrease in left ventricular afterload; and increases in extramural aortic pressure related to the rise in intrathoracic pressure.
Pulse contour analysis
The area under the systolic part of the arterial pressure curve is considered proportional to SV, at least at the aortic level. Using specific peripheral arterial catheters connected to a computer, it is possible to record the area of the systolic part of the arterial pressure curve and therefore to monitor SV, provided that the system has knowledge of the factor of proportionality between SV and the specific curve area. This factor can be determined if SV has been measured by an independent method and stored in memory.
The PiCCO™ device (Pulsion Medical Systems, Munich, Germany) uses the arterial pulse contour method (calibration with transpulmonary thermodilution) and continuously measures and displays stroke volume variation (SVV), which represents the variation in pulse contour SV over a floating period of a few seconds. The LidCO™/PulseCO™ system (LidCO, Cambridge, UK) also uses pulse contour analysis to estimate SV (calibration with lithium dilution) and to calculate and display SVV. It has been demonstrated that SVV (as a marker of respiratory variation in SV) could predict fluid responsiveness in patients receiving mechanical ventilation [32–36].
Limitations
Although the utility of indices related to respiratory changes in arterial pressure to detect preload sensitivity and thus volume responsiveness is indisputable in patients receiving mechanical ventilation, some limitations must be borne in mind. First, these indices cannot be used in patients with spontaneous breathing activity and/or with arrhythmias. Second, it may be hypothesized that, in patients with low lung compliance, the decreased transmission of alveolar pressure to the intrathoracic compartment could result in low ΔPP, even in cases of preload responsiveness. However, high ΔPP may be observed in patients with severe acute lung injury (and thus low lung compliance) [27]. Importantly, low lung compliance is generally associated with high alveolar pressures, even in the case of reduced tidal volume (see below). As a result, despite reduced pressure transmission, the respiratory changes in intrathoracic pressure should remain significant, thus leading to a certain amount of PP variation in preload responsive patients. Overall, the potential role of lung compliance on ΔPP thus remains to be documented.
As a third limitation, de Backer and coworkers [37] recently reported that ΔPP could not predict fluid responsiveness in patients with tidal volume below 8 ml/kg. Others have challenged this viewpoint by arguing that in patients with acute lung injury (in whom reduced tidal volume is recommended), low lung compliance is associated with cyclic changes in both transpulmonary pressure and intrathoracic pressure still high enough for ΔPP to keep its ability to predict fluid responsiveness [38].
Finally, changes in vasomotor tone may modify pulse wave amplification characteristics both by modifying the sites at which pressure wave is reflected and by affecting pulse wave velocity. This may alter the relationship between aortic PP and peripheral PP, and the resulting effect on ΔPP remains to established.
In cases in which it is difficult to interpret the respiratory changes in arterial pressure, it is important to keep in mind that increases in SV or of its surrogates, such as PP [15], during a passive leg raising manoeuvre can be useful to identify patients who are able to respond to volume infusion [24].
Conclusion
In critically ill patients monitored with an arterial catheter, the arterial pressure signal provides the clinician with information that is helpful in decision making. Taking into consideration all of the four arterial pressure values (SAP, DAP, MAP and PP) helps one to define haemodynamic status. In addition, calculation of the respiratory variation in arterial pressure permits reliable prediction of volume responsiveness in patients who are receiving mechanical ventilation. Finally, large-scale studies are needed to test the potential benefits of incorporating ΔPP and/or SVV into protocols for the management of haemodynamically unstable patients.
Abbreviations
- CO:
-
cardiac output
- DAP:
-
diastolic arterial pressure
- MAP:
-
mean arterial pressure
- mRAP:
-
mean right atrial pressure
- PP:
-
arterial pulse pressure
- SAP:
-
systolic arterial pressure
- SV:
-
stroke volume
- SVR:
-
systemic vascular resistance
- SVV:
-
stroke volume variation.
References
Chobanian AV, Bakris GL, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ, the National High Blood Pressure Education Program Coordinating Committee: Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42: 1206-1252. 10.1161/01.HYP.0000107251.49515.c2
Darne B, Girerd X, Safar M, Cambien F, Guize L: Pulsatile versus steady component of blood pressure: a cross-sectional and prospective analysis of cardiovascular mortality. Hypertension 1989, 13: 392-400.
Nichols WW, O'Rourke M: Contours of pressure and flow waves in arteries. In McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical principles. 4th edition. Edited by: Nichols WW, O'Rourke M. London: Oxford University Press; 1998:170-200.
Milnor WR: Hemodynamics. Baltimore: William and Wilkins; 1992.
Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y: Total arterial compliance estimated by the stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol Heart Circ Physiol 1998, 274: H500-H505.
Murgo JP, Westerhof N, Giolma JP, Altobelli SA: Aortic imput impedance in normal man: relationships to pressure waveform. Circulation 1980, 62: 105-115.
O'Rourke MF: Ascending aortic pressure wave indices and cardiovascular disease. Am J Hypertens 2004, 17: 721-723. 10.1016/j.amjhyper.2004.04.002
Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JAE, Glynn RJ: Systolic and diastolic blood pressure, pulse pressure and mean arterial pressure as predictors of cardiovascular risk in men. Hypertension 2000, 36: 801-807.
Michard F, Teboul JL, Richard C, Lecarpentier Y, Chemla D: Arterial pressure monitoring in septic shock. Intensive Care Med 2003, 29: 659.
Berne RM, Levy MN: The arterial system. In Physiology. Edited by: Berne RM, Levy MN. St Louis: Mosby Inc; 1998:415-428.
Chatburn RL: Principles of measurement. In Principles and Practice of Intensive Care Monitoring. Edited by: Tobin MJ. New York: Mc Graw-Hill; 1997:45-61.
Fessler HE, Shade D: Measurement of vascular pressure. In Principles and Practice of Intensive Care Monitoring. Edited by: Tobin MJ. New York: McGraw-Hill; 1997:91-106.
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, et al.: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004, 32: 858-873. 10.1097/01.CCM.0000117317.18092.E4
Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, Heard SO, Martin C, Napolitano LM, Susla GM, et al.: Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med 2004, 32: 1928-1948. 10.1097/01.CCM.0000139761.05492.D6
LeDoux D, Astiz ME, Carpati CM, Rackow EC: Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med 2000, 28: 2729-2732. 10.1097/00003246-200008000-00007
Bourgoin A, Leone M, Delmas A, Garnier F, Albanese J, Martin C: Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med 2005, 33: 780-786. 10.1097/01.CCM.0000157788.20591.23
Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G: Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest 2002, 121: 1245-1252. 10.1378/chest.121.4.1245
Kelly RP, Hayward CS, Avolio AP, O'Rourke MF: Non-invasive determination of age-related changes in the human arterial pulse. Circulation 1989, 80: 1652-1659.
Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D: Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997, 96: 308-315.
McEniery CM, Wallace YS, Maki-Petaja K, McDonnell B, Sharman JE, Retallick C, Franklin SS, Brown MJ, Lloyd RC, Cockroft JR, et al.: Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension 2005, 46: 221-226. 10.1161/01.HYP.0000165310.84801.e0
Perel A, Pizov R, Cotev S: Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology 1987, 67: 498-502.
Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P: Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology 1998, 89: 1313-1321. 10.1097/00000542-199812000-00007
Michard F, Teboul JL: Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care 2000, 4: 282-289. 10.1186/cc710
Teboul JL, Monnet X, Richard C: Arterial pulse pressure variation during positive pressure ventilation and passive leg raising. In Functional Hemodynamic Monitoring. Edited by: Pinsky MR, Payen D. Berlin: Springer-Verlag; 2004:331-343.
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL: Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 2000, 162: 134-138.
Michard F, Teboul JL: Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002, 121: 2000-2008. 10.1378/chest.121.6.2000
Michard F, Chemla D, Richard C, Wysocki M, Pinsky MR, Lecarpentier Y, Teboul JL: Clinical use of respiratory changes in arterial pressure to monitor the hemodynamic effects of PEEP. Am J Respir Crit Care Med 1999, 159: 935-939.
Bendjelid K, Suter PM, Romand JA: The respiratory change in preejection period: a new method to predict fluid responsiveness. J Appl Physiol 2004, 96: 337-342. 10.1152/japplphysiol.00435.2003
Kramer A, Zygun D, Hawes H, Easton P, Ferland A: Pulse pressure variation predicts fluid responsiveness following coronary artery bypass surgery. Chest 2004, 126: 1563-1568. 10.1378/chest.126.5.1563
Vieillard-Baron A, Chergui K, Rabiller A, Peyrouset O, Page B, Beauchet A, Jardin F: Superior vena caval collapsibility as a gauge of volume status in ventilated septic patients. Intensive Care Med 2004, 30: 1734-1739.
Denault YD, Gasior TA, Gorcsan J III, Mandarino WA, Deneault LG, Pinsky MR: Determinants of aortic pressure variation during positive-pressure ventilation in man. Chest 1999, 116: 176-186. 10.1378/chest.116.1.176
Berkenstadt H, Margalit N, Hadani M, Friedman Z, Segal E, Villa Y, Perel A: Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth Analg 2001, 92: 984-989. 10.1097/00000539-200104000-00034
Reuter DA, Felbinger TW, Schmidt C, Kilger E, Goedje O, Lamm P, Goetz AE: Stroke volume variations for assessment of cardiac responsiveness to volume loading in mechanically ventilated patients after cardiac surgery. Intensive Care Med 2002, 28: 392-398. 10.1007/s00134-002-1211-z
Reuter DA, Kirchner A, Felbinger TW, Weis FC, Kilger E, Lamm P, Goetz AE: Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med 2003, 31: 1399-1404. 10.1097/01.CCM.0000059442.37548.E1
Rex S, Brose S, Metzelder S, Huneke R, Schalte G, Autschbach R, Rossaint R, Buhre W: Prediction of fluid responsiveness in patients during cardiac surgery. Br J Anaesth 2004, 93: 782-788. 10.1093/bja/aeh280
Marx G, Cope T, McCrossan L, Swaraj S, Cowan C, Mostafa SM, Wenstone R, Leuwer M: Assessing fluid responsiveness by stroke volume variation in mechanically ventilated patients with severe sepsis. Eur J Anaesthesiol 2004, 21: 132-138. 10.1017/S0265021504002091
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL: Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 2005, 31: 517-523. 10.1007/s00134-005-2586-4
Teboul JL, Vieillard-Baron A: Clinical value of pulse pressure variations in ARDS. Still an unresolved issue? Intensive Care Med 2005, 31: 499-500. 10.1007/s00134-005-2587-3
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
J-LT is a member of the Medical Advisory Board of Pulsion Medical Systems (Germany). BL, DC and CR declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Lamia, B., Chemla, D., Richard, C. et al. Clinical review: Interpretation of arterial pressure wave in shock states. Crit Care 9, 601 (2005). https://doi.org/10.1186/cc3891
Published:
DOI: https://doi.org/10.1186/cc3891