Abstract
Purpose
Properly regulated circadian rhythm supports physical and immunologic function. This rhythm is disrupted in patients with critical illness. We assessed the association between ambient light and circadian melatonin release, measured by urinary 6-sulfatoxymelatonin (6-SMT), in medical intensive care unit (MICU) patients with severe sepsis.
Methods
After excluding patients for renal failure or hepatic failure, blindness, and intracranial disease, seven patients were studied. No environmental manipulation was performed. Urinary 6-SMT specimens were obtained every 4 h. Light was measured in 1-min epochs for two sequential 24-h periods and compared to 6-SMT levels.
Results
No significant differences among urinary 6-SMT levels were found across 4-h time periods or between the 2 days (range 1,190.26 ± 1,040.81–4,738.57 ± 5,543.08 ng, 4-h period p = 0.09, 24-h day p = 0.50). Light levels were low and differed among 4-h periods, but not 24-h averages (minimum 2.32 ± 3.65 lux/min 00:01–04:00, maximum 70.11 ± 79.12 lux/min from 12:01–16:00, 4 h period p = <0.001, 24 h period p = 0.53). There was no relationship between light levels and 6-SMT excretion.
Conclusions
Circadian rhythm was disrupted in patients with severe sepsis, as reflected by disordered diurnal variation of urinary 6-SMT excretion. Light levels were low, exhibited limited diurnal variation, and did not entrain circadian rhythms in these patients.
Similar content being viewed by others
Introduction
Circadian rhythms are disrupted in the medical intensive care unit (MICU) by illness, unregulated noise, patient care interactions, and unregulated light–dark patterns [1–4]. In vivo administration of endotoxin results in disruption of cellular circadian rhythms [5]. Laboratory and clinical studies have demonstrated that disruption of circadian rhythm results in immune system impairment [6, 7], increased incidence and severity of infection [8], and worse outcomes in critically ill patients [9, 10].
Light is the most potent entraining stimulus for circadian rhythm [11]. However, the effect of unregulated light on circadian rhythm of patients in the MICU has not been characterized. Melatonin, a hormone released by the pineal gland, is widely accepted as a marker of circadian rhythm; it is increased during sleepiness and lowest during wakefulness [12]. Serum melatonin or urinary 6-Sulfatoxymelatonin (6-SMT) have been studied previously and are accepted biomarkers of circadian rhythm in critically ill patients [13].
The aim of this study was to assess the relationship between routinely occurring, ambient light and circadian rhythms of patients with severe sepsis by performing a prospective, observational study of ambient light levels in MICU patient care rooms and urinary 6-SMT levels. We hypothesized that ambient light levels in the MICU would maintain a normal circadian relationship with 6-SMT in patients with severe sepsis.
Methods
This study was approved with waiver of consent by the Institutional Review Board of the University of Maryland, Baltimore (Protocol H-29771).
Study design and setting
This was a prospective, observational pilot study of patients admitted to the University of Maryland Medical Center (UMMC) MICU with a diagnosis of severe sepsis in November 2008, February 2009 and March 2009. UMMC is a 705-bed tertiary care teaching hospital with a “closed” model MICU consisting of 29 separate patient rooms, staffed by medical intensivists. Bedside care was provided by critical care registered nurses. There was no specific protocol for environmental regulation with respect to light, noise, and patient—staff interactions, nor were there mandated patient quiet hours or designated sleep times.
The patient care rooms had mean area of 380.7 ± 39.4 sq. ft, each with a window allowing exposure to outside light. The unit was built as a rectangle around central nursing stations. Each room had exposure to ambient light from one of four directions (north, south, east, west).
Patient selection
Eligible patients were between the ages of 18 and 80 years old, with first admissions to the UMMC MICU, and a diagnosis of severe sepsis as defined by the 2001 SCCM/ESICM/ACCP/ATS/SIS Consensus Criteria [14]. Patients were screened for inclusion within 24 h of admission to the MICU.
Patients were excluded if they had a condition resulting in abnormal melatonin production or clearance, including hemodialysis dependent chronic renal failure, cirrhosis or hepatic failure, evidence of renal (admission serum creatinine >1.8 mg/dl or an increase in serum creatinine by 25% in 24 h) or hepatic (transaminases >2 times upper limit of normal) injury, were blind, or had acute intracranial disease (e.g., intracranial hemorrhage or space occupying lesion).
Patients were treated for severe sepsis using a protocol based on the Surviving Sepsis Guidelines [15].
Data collection
Demographic and clinical data were collected from each patient’s medical records. Acuity was assessed using the APACHE II scoring system [16]. After patient enrollment, light levels in the room were measured in 1-min epochs over a 48-h period. Measurements were obtained with an Actitrac actigraph/luxmeter (IM Systems, Baltimore, Maryland) mounted on a central column located at the head of each patient’s bed, in a central location at eye level. Eye closing and opening was spontaneous according to level of consciousness or sedation. In our study, subjects’ eyes were closed spontaneously and not covered with ophthalmic dressings. Measurements were taken continuously over the recording periods, and reported in mean lux/epoch and ranges (lowest to highest measurement in lux). As there was no environmental protocol in place for light control, levels were measured without attempts to control intensity or variation. Patients were admitted to rooms according to patient flow, regardless of patient acuity.
Urine samples were collected from the urinary catheter system every 4 h for a 48-h period beginning from the time of enrollment. Twelve urine samples were obtained for each patient over the 48-h period and analyzed for concentration of 6-SMT, the main melatonin metabolite. Urine volume was also recorded, coinciding with the urine specimen collection. Urine sampling occurred concurrently with continuous light monitoring during the observation period. These samples were stored at −70°C. After collection of samples for the study, the urine specimens were analyzed for 6-SMT using standard ELISA technique (Genway Biotech Inc., San Diego, CA, USA). 6-SMT is secreted and excreted independent of body size, thus no correction for body habitus is necessary [17]. 6-SMT secretion (ng) over each 4-h time interval was calculated as the product of urinary melatonin concentration and urine volume excreted.
Data analysis
Light levels and melatonin concentration measurements were grouped into six time periods: morning (08:01–12:00), day (12:01–16:00), early evening (16:01–20:00), evening (20:01–midnight), night (00:01–04:00), and early morning (04:01–08:00). Light levels were collected continuously in 1-min epochs for 48 h and were averaged over 4-h periods. 6-SMT levels were expressed in nanograms secreted per time interval. Mean light levels and total 6-SMT measurements were compared across periods using two-way analysis of variance (ANOVA) for repeated measures (time and day as independent variables). Measurements were expressed as mean ± standard deviation unless otherwise noted. Patients’ 6-SMT levels were averaged and plotted against light during each of the measured time periods. Linear regression was performed comparing total urinary 6-SMT secretion (dependent variable) and light levels (independent variable). R 2 was calculated to determine the proportion of variance in 6-SMT levels explained by changes in light levels.
Analyses were performed using GB Stat v 9.0 (Silver Spring, MD, USA) using p ≤ 0.05 to denote statistical significance.
Results
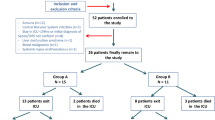
During the enrollment period, 70 patients were admitted for severe sepsis. Fifty-nine were excluded for hepatic or renal failure, or failing to be screened within 24 h of admission (Fig. 1). Of the remaining 11 patients, 3 patients died prior to data collection, and 1 patient was transferred from the MICU prior to completion of data collection; 7 patients remained to be studied. All patients in our cohort had normal renal function on admission to the MICU, which remained consistent during the experimental period, supporting the assumption of stable melatonin elimination rates. All patients enrolled in the study remained in the same room for the duration of the 48-h monitoring period.
Mean age was 63.0 ± 19.5 years. Six patients were African American, and one was white. The mean APACHE II score of patients in our cohort was 24.4 ± 9.4 (Table 1).
A total of 2,880 light measurements were collected for each patient and analyzed. Light levels were found to be highest during daytime hours (40.53 ± 35.27 lux/epoch on day 1 and 70.11 ± 79.12 lux/epoch on day 2) and lowest during night hours (2.32 ± 3.65 lux/epoch on day 1 and 2.59 ± 3.96 lux/epoch on day 2). There was strong evidence that light differed by time period, but not by day (p < 0.001 across time, p = 0.053 across day, Fig. 2).
Total urinary 6-sulfatoxymelatonin secretion and average light levels. Urinary 6-sulfatoxymelatonin measured in nanograms/4-h period and light in lux/minute. Error bars represent standard deviations. Measurements recorded over 48 h divided into 4-h periods. Light levels across time periods had a significant relationship with 4-h period (p < 0.001, two-way ANOVA for repeated measures)
Analysis of 6-SMT levels according to time period and day with two-factor ANOVA demonstrated no significant effects on 6-SMT level (range 1,190.26 ± 1,040.81–4,738.57 ± 5,543.08 ng, 4 h period p = 0.09, 24 h day p = 0.50, Fig. 2).
Linear regression of 6-SMT on light levels showed no appreciable relationship (R 2 = 0.0076, Fig. 3).
Medications affecting melatonin levels are shown in Table 2 [13, 18–20]. Of the medications our patients received, some are known to increase and others to decrease melatonin levels. The most common of these were norepinephrine and phenylephrine. Two of the seven patients studied received sedative medications, which were titrated to maintain a goal Richmond Agitation Sedation Scale (RASS) of -2 [21]. The patients who did not receive sedatives maintained RASS scores between −1 and −5.
Discussion
We found that circadian rhythms, as reflected by urinary 6-SMT levels, were disrupted in patients with severe sepsis. 6-SMT levels failed to exhibit the usual peak in the early morning or nadir during the daytime hours. MICU light levels exhibited diurnal variation but remained low in patient care rooms compared to daytime hours. These light exposures were not associated with entrained circadian rhythms.
To our knowledge, this is the first study to demonstrate that unregulated, naturally occurring ambient light in a MICU environment does not entrain circadian rhythms, as reflected by urinary 6-SMT measurements. Prior studies have demonstrated circadian rhythm disruption as reflected by melatonin levels in septic [11, 22, 23] and non-septic critically ill patients with respiratory failure [24, 25]. Our findings demonstrated loss of circadian rhythmicity of 6-SMT levels consistent with those studies and further assessed concurrent light levels, finding them ineffective as entraining stimuli in the MICU setting.
Urinary 6-SMT level is an accepted metric for measuring circadian rhythm and has been shown to be highly reflective of serum melatonin concentration [26]. Melatonin is a neurohormone released by the pineal gland that is increased during sleepiness and lowest during wakefulness [12]. Although primarily centrally active, melatonin has peripheral activity, including immune system-enhancing effects [27], activity as a free radical scavenger [28], and protective effects against reperfusion injury in gut mucosa [29]. Light has been shown to be effective in suppressing melatonin secretion and entraining circadian rhythms in humans [11, 30, 31]. In normal subjects, peak serum melatonin levels are noted between 1:00 and 3:00 a.m. (>40 pg/ml), and nadirs during the daytime between 10:00 a.m. and 6:00 p.m. (<7 pg/ml) [32], with overall melatonin secretion gradually declining with age [33]. Compared to normal urinary 6-SMT levels our cohort demonstrated an abnormally high secretion of 6-SMT in all time periods, even when taking night into account, when both our patients and normal patients consistently had their eyes closed. One previous study did evaluate circadian rhythm measuring urinary 6-SMT levels concurrently with light levels in patients with respiratory failure, but did not relate these levels to light intensity [34]. 6-SMT levels in that study were low in relation to ours, but also demonstrated loss of diurnal rhythm.
Uncharacteristically high melatonin and subsequent 6-SMT secretion reflects circadian rhythm disruption, likely as a result of critical illness in combination with overall low light levels. It has been shown that other critical illnesses, such as myocardial infarction [35], traumatic brain injury [9], and surgical and medical ICU delirium [36, 37], are associated with circadian rhythm and sleep-wake cycle disruption, but until now little work has documented MICU light levels in relationship to circadian rhythm disruption.
Although our finding that circadian rhythm was disrupted in patients with severe sepsis is consistent with prior work, the lack of association between room light levels and urinary 6-SMT excretion is unexpected. Two possible explanations are to be considered: first, light levels present in the patient rooms may have lacked the intensity or duration to influence circadian rhythm. Also, it is possible that light as a circadian rhythm entraining force may not influence patients with severe sepsis, as the melatonin overproduction may be a physiologic response to critical illness.
When using light therapy to treat disorders such as seasonal affective disorder or jet lag, typically 30–60 min of 5,000 to 10,000 lux exposure is administered [38]. Although circadian rhythm entrainment has been demonstrated in healthy individuals with extended exposure to as little as 180 lux [39], a study conducted by Perras found that exposure to 1 h of 10,000 lux failed to influence circadian rhythms in a critically ill population with respiratory failure [22]. The intensity of light exposure experienced by our patients was far lower than 10,000 lux, with the highest intensity being approximately 650 lux. Thus, the light intensity experienced by our patients was lower than the therapeutic levels used in psychiatric disorders and in entraining circadian rhythms in prior studies performed on critically ill patients. Although the overall light intensity was low relative to therapeutic exposure, the light exposure experienced by our patients maintained a diurnal variation, in addition to peaks of moderate intensity (650 lux). Despite this circadian pattern and magnitude, such exposure was not sufficient to entrain 6-SMT secretion in our cohort.
It is also possible that light is ineffective in entraining circadian rhythms in patients with severe sepsis. Circadian rhythm is disrupted in animal models exposed to endotoxin [40] and humans with severe sepsis [10]. As noted, prior human studies have demonstrated that intense, but limited light exposure in the early morning period has little effect on urinary melatonin [22]. Thus, severe sepsis itself may be a pathology characterized by circadian rhythm disruption, refractory to short bursts of timed light as an entraining force. Prior to ours, no study has evaluated the effect of normally occurring, ambient light on the circadian rhythm of individuals with severe sepsis.
Further circadian rhythm disruption may have been precipitated or perpetuated by the unscheduled treatments and interactions conducted by patient care personnel occurring around the clock [4]. Certain medications commonly used in treating the critically ill could have affected overall melatonin secretion (Table 2), specifically adrenergic infusions [13]. These infusions result in an increase of melatonin secretion while administered. Potential disruption of cyclic melatonin release can result from continuous vasoactive infusions.
The evaluation of urinary 6-SMT is predicated on normal renal and hepatic function. We excluded patients with chronic insufficiency or frank failure of these organs to avoid misinterpretation of impaired metabolism of melatonin as increased secretion. Unrecognized, subclinical organ impairment influencing these values could diminish sensitivity to normal circadian relationships but not, in our opinion, to the extent that we observed.
It is possible that the seasonality of our study could have affected our results. Our light measurements were obtained in the winter months. Comparing winter to summer periods, total time of bright light exposure differs when measuring intensities greater than 1,000 lux [41]. Thus, it is possible that entrainment might be observed in other seasons. Additionally, we evaluated natural light levels only in our MICU rooms; findings might be different in other MICU settings, characterized by different light levels, patient populations, and medication practices.
Conclusion
This study illustrates circadian rhythm disruption, as represented by high levels of urinary 6-SMT secretion that varied no more than could be expected by chance over 4-h periods, in patients with severe sepsis. MICU patient room light levels were not associated with circadian rhythm entrainment. Since proper circadian rhythm and the associated sleep-wake cycle are important, future study of regulated light in higher intensities and longer durations and its association with circadian rhythm is warranted. A better understanding of this relationship and the possible impact of environmental protocols on circadian rhythm may result in improvement in patient care and clinical outcomes.
References
Hilton BA (1976) Quantity and quality of patients’ sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs 1:453–468
Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ (2001) Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med 163:451–457
Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, Hanly PJ (2003) Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med 167:708–715
Tamburri LM, DiBrienza R, Zozula R, Redeker NS (2004) Nocturnal care interactions with patients in critical care units. Am J Crit Care 13:102–112
Haimovich B, Calvano J, Haimovich AD, Calvano SE, Coyle SM, Lowry SF (2010) In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Crit Care Med 38:751–758
Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC (1996) Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J 10:643–653
Spiegel K, Sheridan JF, Van Cauter E (2002) Effect of sleep deprivation on response to immunization. JAMA 288:1471–1472
Everson CA, Toth LA (2000) Systemic bacterial invasion induced by sleep deprivation. Am J Physiol Regul Integr Comp Physiol 278:R905–R916
Valente M, Placidi F, Oliveira AJ, Bigagli A, Morghen I, Proietti R, Gigli GL (2002) Sleep organization pattern as a prognostic marker at the subacute stage of post-traumatic coma. Clin Neurophysiol 113:1798–1805
Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, Ferti L, Siostrzonek P (2002) Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med 30:536–540
Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sánchez R, Ríos CD, Freitag WO, Richardson GS, Kronauer RE (1986) Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science 233:667–671
Lewy AJ, Sack RL, Miller LS, Hoban TM, Singer CM, Samples JR, Krauss GL (1986) The use of plasma melatonin levels and light in the assessment and treatment of chronobiologic sleep and mood disorders. J Neural Transm Suppl 21:311–322
Bourne RS, Mills GH (2006) Melatonin: possible implications for the postoperative and critically ill patient. Intensive Care Med 32:371–379. doi:10.1007/s00134-005-0061
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29:530–538. doi:10.1007/s00134-003-1662
Dellinger RP, Levy MM, Carlet JM et al (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Bojkowski CJ, Arendt J (1990) Factors influencing urinary 6-sulphatoxymelatonin, a major melatonin metabolite, in normal human subjects. Clin Endocrinol 33:435–444
Utiger RD (1992) Melatonin-the hormone of darkness. N Engl J Med 327:1377–1379
Barassin S, Kalsbeek A, Saboureau M, Bothorel B, Vivien-Roels B, Malan A, Buijs RM, Pevet P (2000) Potentiation effect of vasopressin on melatonin secretion as determined by trans-pineal microdialysis in the rat. J Neuroendocrinol 12:61–68
Dispersyn G, Pain L, Touitou Y (2010) Propofol anesthesia significantly alters plasma blood levels of melatonin in rats. Anesthesiology 112:333–337
Sessler CN, Gosnell M, Grap MJ, Brophy GT, O’Neal PV, Keane KA, Tesoro EP, Elswick RK (2002) The Richmond agitation-sedation scale: validity and reliability in adult intensive care patients. Am J Respir Crit Care Med 166:1338–1344
Perras B, Meier M, Dodt C (2007) Light and darkness fail to regulate melatonin release in critically ill humans. Intensive Care Med 33:1954–1958. doi:10.1007/s00134-007-0769
Perras B, Kurowski V, Dodt C (2006) Nocturnal melatonin concentration is correlated with illness severity in patients with septic disease. Intensive Care Med 32:624–625. doi:10.1007/s00134-006-0069
Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, Balaum H, Shenkman L (1999) Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci 317:278–281
Olofsson K, Alling C, Lundberg D, Malmros C (2004) Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand 48:679–684
Bojkowski CJ, Arendt J, Shih MC, Markey SP (1987) Melatonin secretion in humans assessed by measuring its metabolite, 6-sulfatoxymelatonin. Clin Chem 33:1343–1348
Maestronin GJ, Conti A, Pierpaoli W (1989) Melatonin, stress, and the immune system. Pineal Res Rev 7:203–226
Tan DX, Chen L-D, Poeggeler B, Manchester LC, Reiter RJ (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J 1:57–60
Kato K, Asai S, Murai I, Nagata T, Takahashi Y, Komuro S, Iwasaki A, Ishikawa K, Arakawa Y (2001) Melatonin’s gastroprotective and antistress roles involve both central and peripheral effects. J Gastroenterol 36:91–95
Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP (1980) Light suppresses melatonin secretion in humans. Science 210:1267–1269
Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C (2000) Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 526(Pt 3):695–702
Crasson M, Kjiri S, Colin A, Kjiri K, L’Hermite-Baleriaux M, Ansseau M, Legros JJ (2004) Serum melatonin and urinary 6-sulfatoxymelatonin in major depression. Psychoneuroendocrinology 29:1–12. doi:10.1016/S0306-4530(02)00123-3
Reiter RJ (1995) The pineal gland and melatonin in relation to aging: a summary of the theories and of the data. Exp Gerontol 30:192–212
Frisk U, Olsson J, Nylen P, Hahn RG (2004) Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci (Lond) 107:47–53
Broughton R, Baron R (1978) Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr Clin Neurophysiol 45:348–360
Yildizeli B, Ozyurtkan MO, Batirel HF, Kuşcu K, Bekiroğlu N, Yüksel M (2005) Factors associated with postoperative delirium after thoracic surgery. Ann Thorac Surg 79:1004–1009
Weinhouse GL, Schwab RJ, Watson PL, Patil N, Vaccaro B, Pandharipande P, Ely EW (2009) Bench-to-bedside review: Delirium in ICU patients—importance of sleep deprivation. Crit Care 13:234. doi:10.1186/cc8131
Shirani A, St Louis EK (2009) Illuminating rationale and uses for light therapy. J Clin Sleep Med 5:155–163
Boivin DB, Czeisler CA (1998) Resetting of circadian melatonin and cortisol rhythms in humans by ordinary room light. Neuroreport 9:779–782
Okada K, Yano M, Doki Y, Azama T, Iwanaga H, Miki H, Nakayama M, Miyata H, Takiguchi S, Fujiwara Y, Yasuda T, Ishida N, Monden M (2008) Injection of LPS causes transient suppression of biological clock genes in rats. J Surg Res 145:5–12
Hebert M, Dumont M, Paquet J (1998) Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol Int 15:59–70
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verceles, A.C., Silhan, L., Terrin, M. et al. Circadian rhythm disruption in severe sepsis: the effect of ambient light on urinary 6-sulfatoxymelatonin secretion. Intensive Care Med 38, 804–810 (2012). https://doi.org/10.1007/s00134-012-2494-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2494-3