Abstract
Delirium occurs frequently in critically ill patients and has been associated with both short-term and long-term consequences. Efforts to decrease delirium prevalence have been directed at identifying and modifying its risk factors. One potentially modifiable risk factor is sleep deprivation. Critically ill patients are known to experience poor sleep quality with severe sleep fragmentation and disruption of sleep architecture. Poor sleep while in the intensive care unit is one of the most common complaints of patients who survive critical illness. The relationship between delirium and sleep deprivation remains controversial. However, studies have demonstrated many similarities between the clinical and physiologic profiles of patients with delirium and sleep deprivation. This article aims to review the literature, the clinical and neurobiologic consequences of sleep deprivation, and the potential relationship between sleep deprivation and delirium in intensive care unit patients. Sleep deprivation may prove to be a modifiable risk factor for the development of delirium with important implications for the acute and long-term outcome of critically ill patients.
Similar content being viewed by others
Introduction
Delirium is common in critical care units, particularly in older people. It is a clinical syndrome characterized by acute onset of fluctuating disturbance in consciousness, inattention, and cognitive dysfunction. Patients may be agitated (hyperactive delirium), may be withdrawn (hypoactive or quiet delirium), or may have features of both agitation and withdrawal at times [1]. Delirium has been shown to occur in up to 80% of critically ill patients and to be an independent predictor of adverse intensive care unit (ICU) outcomes, including increased risk of death, longer hospital stay, and higher costs [2–7].
Epidemiologic studies have identified numerous risk factors for the development of delirium. Advanced age, pre-existing cognitive impairment, electrolyte disturbance, and many frequently used ICU medications such as benzodiazepines are among the usually cited factors associated with delirium. Sleep deprivation, common in ICU patients, may also be a contributing factor.
Hospitalized patients, particularly those who are critically ill, are known to have severe sleep fragmentation and disturbed sleep. The sleep typical of a critically ill patient is characterized by a predominance of wakefulness and light sleep (sleep stages I and II), and a relative lack of rapid eye movement (REM) and deep sleep (delta sleep, formerly referred to as non-REM sleep stages III/IV) [8–11]. Sleep deprivation is known to lead to several clinical and physiologic manifestations also found in delirium; however, its role in the development of ICU delirium is controversial.
This article will review the clinical and neurophysiologic similarities between delirium and sleep deprivation. The importance of this association lies in its potential as another target for intervention to prevent acute ICU delirium.
Clinical similarities between delirium and sleep deprivation
The central components of delirium - that is, inattention, fluctuating mental status, and cognitive dysfunction - are also characteristic of patients with sleep deprivation [12, 13]. Studies of the effects of sleep loss have mostly included healthy volunteers, and the studies apply models of sleep loss that may not be directly applicable to the critically ill patient; regardless, these sleep-deprived individuals have shown all of the clinical manifestations of hypoactive (quiet) delirium. Sleepiness is the most consistent behavioral consequence of sleep deprivation, with drowsiness and pressure to fall asleep (microsleeps) increasing with sleep loss. Negative effects on mood, loss of vigor, fatigue, and impaired cognition have also been observed after all forms of experimental sleep deprivation [14]. A tendency toward psychotic behavior with associated paranoia occurred in a few subjects deprived of sleep for 112 hours [15]. It was noted that the psychotic behavior increased during the night and abated during the day, as is also typical of ICU delirium. Attention and memory impairment, two key elements in the diagnosis of delirium, have also been found after periods of total and partial sleep deprivation [16, 17].
The association of sleep deprivation and hyperactive delirium is more controversial. Sleep deprivation, as studied in healthy volunteers, has not been shown to lead to the overt agitation or hallucinations present with this delirium subtype. In 1959 a New York disc jockey stayed awake for 200 hours [18, 19]. Toward the end of the 200 hours he developed paranoid delusions and auditory and visual hallucinations, and these problems were initially attributed to sleep deprivation. Later, it was suspected that the large doses of methylphenidate that the disc jockey took to stay awake were the actual cause of his paranoia and hallucinations.
Dement and Vaughan studied the effects of prolonged wakefulness, and observed that healthy volunteers who were sleep deprived would become confused, ill-tempered, and extremely sleepy; however, they never became either psychotic or hyperactively delirious [20]. The longest observed case of sleep deprivation involved an 18 year old who stayed awake for 264 hours. At times during his long-term sleep deprivation, he would become angry that he was not being allowed to fall asleep. He was not, however, reported to experience symptomatology consistent with hyperactive delirium or hallucinations [21].
Sleep loss, as with delirium, has been shown to result in demonstrable neurocognitive dysfunction. Sleep deprivation leads to decrements in psychomotor vigilance (the ability to sustain attention and respond in an appropriate amount of time), working memory, and response inhibition (subjects respond when no stimulus is present or respond to the wrong stimulus) [22]. Problems with verbal fluency, creative thinking, nonverbal planning, and temporal memory, as may be observed in delirium, may also develop as a result of sleep deprivation [13]. Even one night of total sleep deprivation reduces the alertness of healthy volunteers and their performance on serial addition/subtraction tasks [23].
The persistence of these adverse changes in cognitive performance is often underestimated. Mood disturbance and subjective sleepiness return to baseline quickly once recovery sleep begins; however, some performance tasks are slower to recover [24]. Healthy volunteers sleep deprived for up to 48 hours, for example, have a continued abnormality in psychomotor vigilance even after 5 nights of recovery sleep [25]. A similar delay in psychomotor recovery occurs with delirium. Patients who were delirious in the hospital are observed to recover to their baseline mental status prior to discharge and may even return to work, but with demonstrable impairments in task performance and thought processing when tested months later [26].
Exploring the mechanistic relationship between delirium and sleep deprivation
Delirium is believed to be due to a malfunction of specific regions of the cerebral cortex and related structures of the brainstem. This malfunction may be due to vulnerability of distinct neural circuits to a variety of insults leading to cellular dysfunction. Two interconnecting neural circuits - one involving the prefrontal cortex, anterior cingulate, and basal ganglia; and the other circuit involving the parietal lobes, superior colliculus, and thalamic pulvinar - have been proposed as important pathways for attention and working memory [14]. When these neural circuits are compromised, the resulting imbalance of the involved neurotransmitters is thought to lead to the clinical manifestations of delirium [27]. Although multiple neurotransmitter systems are probably involved, it is thought that a deficiency in cholinergic innervation and excess of dopaminergic stimulation are the final common pathways for the development of clinical signs and symptoms of delirium [28]. Once thought to be just temporary, the cognitive manifestations of this neuronal dysfunction have been shown to last from months to years in survivors of critical illness [29, 30].
Evidence of the importance of the prefrontal cortex and non-dominant posterior parietal cortex comes from neurophysiologic studies and is supported by functional imaging and lesion studies. Mesulam and colleagues and Koponen and colleagues both identified lesions with a prefrontal and a posterior parietal focus in patients with delirium [31, 32]. Other investigators have identified lesions of the caudate or thalamus, areas that directly interact with the prefrontal cortex or posterior parietal cortex, in delirious patients [33]. Consistent with these findings are positron emission tomography and single-photon emission computerized tomography studies of some delirious patients demonstrating changes of cerebral perfusion and metabolism in these same regions of the central nervous system [34, 35].
Sleep deprivation has been shown to affect the same regions of the central nervous system. Thomas and colleagues, for example, measured the regional cerebral metabolic rate by positron emission tomography scan in healthy volunteers deprived of sleep for 24 hours [23]. These subjects had a global decrease in glucose metabolism but with focally accentuated decreases in glucose uptake in the prefrontal cortex, thalamus, and posterior parietal cortex in response to cognitive tasks. Interestingly, blood oxygen level-dependent functional magnetic resonance imaging studies of healthy subjects deprived of sleep for 35 hours demonstrated an activation of the prefrontal and parietal cortices during a specific learning task [36]. The authors postulate that this phenomenon represents compensation for the failure of normal neural systems when challenged with specific learning tasks. Electroencephalography studies of sleep-deprived subjects similarly support the theory that frontal and parietal cortical areas may be susceptible to sleep deprivation [37].
Sleep deprivation, as is observed in ICU patients, also shares some of the neurohormonal changes observed in delirium. It has long been known that the cholinergic system is integral to the generation of REM sleep. Evidence for a relationship between REM sleep deprivation and cholinergic dysregulation derives mostly from animal models that suggest acetylcholine levels would decrease in the brain after REM sleep deprivation, as may occur in delirium [38, 39].
Dopamine is believed to be important to attention, motor activity, mood, motivation, and memory [28]. Dopamine and acetylcholine interact closely, often reciprocally, in the central nervous system [27]. Dopamine levels are increased under conditions known to cause delirium; that is, intoxication with 3,4-dihydroxy-L-phenylalanine and opiates, cocaine binges, and hypoxia [40]. Activation of the dopaminergic system is also observed after periods of sleep deprivation [41].
The mechanism of delirium and the consequences of sleep deprivation continue to be studied. Although a direct relationship has not been established, it seems probable that these two conditions that share so many epidemiologic, biochemical, and anatomic similarities would be clinically related. Table 1 summarizes the similarities between delirium and sleep deprivation.
Sleep deprivation as a risk factor for delirium
Patients are at higher risk for developing delirium if they are older and have pre-existing cognitive impairment, sensory impairment, poor functional status, immobility, multiple co-morbid medical problems, alcohol abuse, depression, and cancer. The risk of developing delirium is further enhanced by the addition of precipitating factors imposed both as part of the underlying illness and of treatment. A common precipitating factor in ICU patients is the administration of certain medications such as benzodiazepines [42]; even routine practices such as bladder catheterization and restraints, however, may be enough to lead to clinically apparent delirium.
Is sleep deprivation a risk factor for delirium? Few clinical studies have been designed with the primary intent of answering this question. Helton and colleagues studied 62 critically ill medical and surgical patients during their first 5 days in the ICU with the intention of correlating patients' sleep deprivation with the development of mental status changes [43]. The authors did attempt to control for medications and conditions that would affect mental status. Sleep deprivation was determined by the number of uninterrupted sleep cycles - that is, 75-minute periods of time without interruption - compared with what would be normal for the patient at home. The authors were therefore measuring potential sleep cycles. They found that mental status changes were more likely in patients with greater sleep deprivation. To fully establish this relationship, however, more rigorous investigation utilizing polysomnography and objective measures of delirium in critically ill patients are needed.
Investigation of the relationship between sleep deprivation and delirium
To carefully study the effect of sleep deprivation on the development of ICU delirium, polysomnography (PSG) needs to be performed over a 24-hour period. Sleep in critically ill patients is distributed irregularly throughout the day and night [8, 10, 11]. To fully evaluate the effects of sleep on clinical outcomes such as delirium, PSG cannot be limited to the nocturnal time period and should include as much time during the patient's ICU stay as possible. In addition, vasoactive medications, sedatives, and analgesics all have profound effects on sleep architecture [44]. As these medications are weaned, patients' sleep patterns will also change.
Moreover, the interpretation of PSG in the ICU is challenging and is a new frontier in critical care. In fact, the traditional, manual scoring system for PSG may not be as reproducible as spectral analysis of electroencephalography in mechanically ventilated critically ill patients [45]. Encephalopathy is common in the ICU and can cause electroencephalography patterns similar to slow-wave sleep [10]. Sedatives can also cause profound effects on the electroencephalography, producing a decrease in electroencephalography amplitude and an increase in frequency - which may be erroneously scored as wakefulness if using standard Rechtschaffen and Kales criteria. Short periods of electroencephalography burst suppression may also occur secondary to benzodiazepines and propofol administration on PSG epochs otherwise scored as stage I or stage II sleep. It is important to take into account these electroencephalography findings in addition to standard sleep staging when interpreting PSG in ICU patients.
Independent of PSG data, risk factors for the development of sleep deprivation in critically ill patients overlap with those of delirium (Table 2). Admission to an ICU alone, mechanical ventilation, pain, and stress are risk factors for the development of both delirium and sleep deprivation. Patients with pre-existing cognitive impairment (that is, dementia) are both at high risk for developing delirium and are also known to have sleep problems at baseline with features similar to those of the critically ill. Patients with dementia have an increased percentage of stage I sleep, decreased slow-wave sleep, loss of the defining features of stage II sleep (sleep spindles and K complexes), and decreased REM percentage - all of which are features of the poor sleep of many critically ill patients.
In addition, many of the commonly used medications associated with delirium - benzodiazepines and opiates, for example-adversely affect sleep architecture by suppressing both slow-wave sleep and REM sleep [44]. Those medications with an anticholinergic effect, if they cross the blood-brain barrier, are highly associated with delirium; and these medications may also have characteristic effects on sleep [46]. Medications with a significant anticholinergic effect, such as tricyclic antidepressants and antihistamines, suppress REM sleep. Other medications commonly used in the ICU and associated with delirium - such as corticosteroids, sympathomimetics, and anticonvulsants - also disrupt sleep [44].
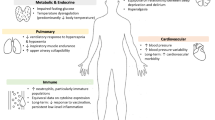
In conclusion, delirium may be caused by numerous and protean insults to peripheral, systemic, and central nervous system function leading to the same final common pathway. It is in this context that sleep deprivation is plausible as a contributing factor in the onset of delirium even in the absence of data definitively establishing it as an independent risk factor (Figure 1).
Outcomes of delirium
The importance of diagnosing and treating delirium is related to its association with adverse outcomes. Numerous studies have confirmed that the development of delirium during a hospital course is associated with increased length of stay in the hospital [7], worse physical and cognitive status upon discharge and for at least 12 months thereafter [26], and a higher mortality [2, 4, 47]. All clinical delirium variants (that is, hyperactive and hypoactive) have been associated with poorer outcomes relative to those who do not develop delirium [47]. Delirium has also been demonstrated to cause distress among family members and caretakers as well as among those patients with recall of their experience. Patients who developed delirium while in the hospital were more likely to require institutional care following their acute illness, to suffer a decline in their prehospitalization performance status, and to have higher 1-year mortality then a comparable group who did not develop delirium during hospitalization.
Poor neurocognitive outcomes of patients who developed delirium but survived their critical illness have also been demonstrated [29, 30]. Some of these patients have demonstrable, long-lasting, possibly permanent deficits after discharge from the hospital. Several investigators have found a higher incidence of dementia in follow-up of patients who became delirious while in the hospital [48]. It is not known, however, whether the delirium itself was the cause, whether delirium exposed a pre-existing tendency toward cognitive impairment, or whether other variables such as medications were responsible for this phenomenon.
The development of delirium is clearly associated with poor short-term and long-term outcomes relative to those patients who do not develop delirium. It is far less clear how to intercede in such a way as to positively affect outcomes. If sleep deprivation does ultimately prove to be a cause of or a contributor to ICU delirium, it would establish its link with these poor ICU outcomes and would intensify the need to create and study intervention protocols designed to facilitate sleep in the ICU.
Management of sleep deprivation
In light of available data, it may be reasonable to conclude that the treatment of sleep deprivation may prevent, shorten, or improve delirium and its medical consequences. Similar to the treatment of delirium, an integrative approach with both pharmacologic and nonpharmacologic strategies should be undertaken to treat sleep deprivation [44]. As with delirium, attention to controlling the patients' environment should include maintaining a quiet, dark room during the night, and reducing sleep interruptions during the nocturnal hours. During the day, however, light levels should be maintained and patients kept awake if possible as one strategy to consolidate sleep at night.
A review of possible pre-existing sleep disorders and medications that could disrupt sleep should routinely be conducted (Table 2). For patients who require mechanical ventilation, attempts should be made to minimize discomfort, to optimize patient-ventilator synchrony, and to attend to possible central apneas that may occur on pressure support ventilation and proportional assist ventilation as these have also been shown to disrupt sleep [49, 50]. For those patients still unable to sleep despite nonpharmacologic means, short-acting hypnotics and sedating antipsychotics and anti- depressant medications have been used off-label for this purpose but increase the patients' risk of developing delirium. For those patients who need continuous sedation (that is, those on mechanical ventilation), nocturnal propofol may be a reasonable agent to use - based on some animal studies suggesting that resolution of sleepiness known to occur under natural sleep may also occur under sedation with propofol [51, 52]. Studies need to be performed, however, to demonstrate that treating sleep deprivation in ICU patients improves delirium emergence or duration.
Conclusion
Delirium is highly prevalent among critically ill patients. Its causes are protean but its manifestations represent dysfunction of specific neurohormonal pathways of the central nervous system. Severe sleep deprivation is an important problem for critically ill patients as it has been shown to have both short-term and long-term effects on patients' quality of life. Sleep deprivation may also be a risk factor for delirium, which would further link it to higher morbidity, mortality, and length of ICU stay. Sleep deprivation research has revealed many similarities, both clinically as well as experimentally, with delirium. These similarities are characterized by poor thought processing, attention and memory deficits, and fluctuating mental status. These findings are consistent with the alterations found on specific regional brain activity both by lesion studies as well as by functional imaging studies that implicate the prefrontal and the nondominant parietal cortices. Animal experiments and some indirect evidence from human studies suggest a prominent role for the cholinergic and dopaminergic systems, but clearly the neuropathogenesis of delirium and the consequences of sleep deprivation are complicated and probably involve multiple overlapping neurotransmitter circuits and neurohormonal pathways.
The occurrence of delirium has both short-term implications for patients' acute illness as well as long-term implications for patients' recovery and subsequent quality of life. A paucity of sleep for these same critically ill patients denies them an integral homeostatic biologic function essential for life and with putatively restorative function. Sleep deprivation in the ICU is profound and may be a risk factor for delirium. It is important for clinicians to realize, however, that - even without an established link to delirium - sleep deprivation itself is a potentially treatable cause of significant patient discomfort with an established link to adverse ICU quality of life. Accordingly, treatment of sleep deprivation should be considered a comfort measure comparable to pain control and anxiolysis.
It seems likely from the available data, although unproven, that sleep deprivation may play an important role in the pathogenesis of some cases of delirium by affecting those areas of the central nervous system associated with delirium. Prevention or treatment of sleep deprivation may help to prevent or improve ICU delirium and its consequences, but further research is needed to determine the exact role sleep deprivation plays in its pathogenesis.
Abbreviations
- ICU:
-
intensive care unit
- PSG:
-
polysomnography
- REM:
-
rapid eye movement.
References
Peterson JF, Pun BT, Dittus RS, Thomason JWW, Jackson JC, Shintani AK, Ely EW: Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc 2006, 54: 479-484. 10.1111/j.1532-5415.2005.00621.x
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, Inouye SK, Bernard GR, Dittus RS: Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004, 291: 1753-1762. 10.1001/jama.291.14.1753
Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y: Incidence, risk factors and consequences of ICU delirium. Intensive Care Med 2007, 33: 66-73. 10.1007/s00134-006-0399-8
Lin S, Liu C, Wang C, Lin H, Huang C, Huang P, Fang Y, Shieh M, Kuo H: The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med 2004, 32: 2254-2259. 10.1097/01.CCM.0000110878.49476.42
Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, Truman B, Bernard GR, Dittus RS, Ely EW: Costs associated with delirium in mechanically ventilated patients. Crit Care Med 2004, 32: 955-962. 10.1097/01.CCM.0000119429.16055.92
Thomason JWW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW: Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care 2005, 9: R375-R381. 10.1186/cc3729
Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK: The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med 2001, 27: 1892-1900. 10.1007/s00134-001-1132-2
Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ: Sleep in critically ill patients requiring mechanical ventilation. Chest 2000, 117: 809-818. 10.1378/chest.117.3.809
Aurell J, Elmqvist D: Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985, 290: 1029-1032. 10.1136/bmj.290.6474.1029
Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ: Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med 2001, 163: 451-457.
Gabor J, Cooper A, Crombach S, Lee B, Kadikar N, Bettger HE, Hanly PJ: Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med 2003, 167: 708-715. 10.1164/rccm.2201090
Dinges DF, Kribbs NB: Performing while sleepy: effects of experimentally-induced sleepiness. In Sleep, Sleepiness and Performance. Edited by: Monk TH. New York: J Wiley; 1991:97-128.
Harrison Y, Horne JA: Prefrontal neuropsychological effects of sleep deprivation in young adults - a model for healthy aging? Sleep 2000, 23: 1067-73.
Durmer JS, Dinges DF: Neurocognitive consequences of sleep deprivation. Semin Neurol 2005, 25: 117-125. 10.1055/s-2005-867080
Tyler DB: Psychological changes during experimental sleep deprivation. Dis Nerv Syst 1955, 16: 293-299.
Mograss MA, Guillem F, Brazzini-Poisson V, Godbout R: The effects of total sleep deprivation on recognition memory processes: a study of event-related potential. Neurobiol Learn Mem 2009, 91: 343-352. 10.1016/j.nlm.2009.01.008
Fisher S: The microstructure of dual-task interaction: sleep deprivation and the control of attention. Perception 1980, 9: 327-337. 10.1068/p090327
Man From Mars Production: Peter Tripp Marathon[http://man-frommars.com/tripp.html]
Sleep Deprivation: It's More then Dark Circles Under the Eyes[http://www.pbs.org/livelihood/nightshift/sleep_deprivation.html]
Dement WC, Vaughan C: The Promise of Sleep. New York: Delacorte Press; 1999.
Gulevich G, Dement W, Johnson L: Psychiatric and EEG observations on a case of prolonged (264 hours) wakefulness. Arch Gen Psychiatry 1966, 15: 29-35.
Dorrian J, Rogers NL, Dinges DF: Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In Sleep Deprivation: Clinical Issues, Pharmacology, and Sleep Loss Effects. Volume 193. Edited by: Kushida CA. New York: Marcel Dekker; 2005:39-70.
Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H Jr, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D: Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 2000, 9: 335-352. 10.1046/j.1365-2869.2000.00225.x
Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ: Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 2003, 12: 1-12. 10.1046/j.1365-2869.2003.00337.x
Lamond N, Jay SM, Dorrian J, Ferguson SA, Jones C, Dawson D: The dynamics of neurobehavioural recovery following sleep loss. J Sleep Res 2007, 16: 33-41. 10.1111/j.1365-2869.2007.00574.x
McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F: Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ 2001, 165: 575-583.
Trzepacz PT: Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord 1999, 10: 330-334. 10.1159/000017164
Trzepacz PT: Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry 2000, 5: 132-148.
Hopkins RO, Weaver LK, Pope D, Orme JF Jr, Bigler ED, Larson-Lohr V: Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 1999, 160: 50-56.
Jackson JC, Hart RP, Gordon SM, Shintani A, Truman B, May L, Ely EW: Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med 2003, 31: 1226-1234. 10.1097/01.CCM.0000059996.30263.94
Mesulam MM, Waxman SG, Geschwind N, Sabin TD: Acute confusional states with right middle cerebral artery infarctions. J Neurol Neurosurg Psychiatry 1976, 39: 84-89. 10.1136/jnnp.39.1.84
Koponen H, Hurri L, Stenback U, Mattila E, Soininen H, Riekkinen PJ: Computed tomography findings in delirium. J Nerv Ment Dis 1989, 177: 226-231. 10.1097/00005053-198904000-00006
Bogousslavsky J, Ferranzzini M, Regli E, Assal G, Tanabe H, Delaloye-Bischof A: Manic delirium and frontal-like syndrome with paramedian infarction of right thalamus. J Neurol Neurosurg Psychiatry 1988, 51: 116-119. 10.1136/jnnp.51.1.116
Doyle M, Warden D: Use of SPECT to evaluate postcardiotomy delirium. Am J Psychiatry 1996, 153: 838-839.
Lerner MD, Rosenstein DL: Neuroimaging in delirium and related conditions. Semin Clin Neuropsychiatry 2000, 5: 98-112.
Drummond SPA, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB: Altered brain response to verbal learning following sleep deprivation. Nature 2000, 403: 655-657. 10.1038/35001068
Smith ME, McEvoy LK, Gevins A: The impact of moderate sleep loss on neurophysiologic signals during working memory task performance. Sleep 2002, 25: 784-789.
Benedito MAC, Camarini R: Rapid eye movement sleep deprivation induces an increase in acetylcholinesterase activity in discrete rat brain regions. Braz J Med Biol Res 2001, 34: 103-109. 10.1590/S0100-879X2001000100012
Tsai LL, Bergmann BM, Perry BD, Rechtschaffen A: Effects of chronic sleep deprivation on central cholinergic receptors in rat brain. Brain Res 1994, 642: 95-103. 10.1016/0006-8993(94)90909-1
Wetli CV, Mash D, Karch SB: Cocaine-associated agitated delirium and the neuroleptic malignant syndrome. Am J Emerg Med 1996, 14: 425-428. 10.1016/S0735-6757(96)90066-2
Ebert D, Albert R, Hammon G, Strasser B, May A, Merz A: Eye-blink rates and depression. Is the antidepressant effect of sleep deprivation mediated by the dopamine system? Neuropsychopharmacology 1996, 15: 332-339. 10.1016/0893-133X(95)00237-8
Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, Bernard GR, Ely EW: Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 2006, 104: 21-26. 10.1097/00000542-200601000-00005
Helton MC, Gordon SH, Nunnery SL: The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung 1980, 9: 464-468.
Bourne RS, Mills GH: Sleep disruption in critically ill patients-pharmacologic considerations. Anesthesia 2004, 59: 374-384. 10.1111/j.1365-2044.2004.03664.x
Ambrogio C, Koebnick J, Quan SF, Ranieri VM, Parthasarathy S: Assessment of sleep in ventilator-supported critically ill patients. Sleep 2008, 31: 1559-1568.
Pandharipande P, Ely EW: Sedative and analgesic medications: risk factors for delirum and sleep disturbances in the critically ill. Crit Care Clin 2006, 22: 313-327. 10.1016/j.ccc.2006.02.010
Marcantonio E, Ta T, Duthie E, Resnick NM: Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc 2002, 50: 850-857. 10.1046/j.1532-5415.2002.50210.x
Rahkonen T, Luukkainen-Markkula R, Paanilla S, Sivenius J, Sulkava R: Delirium episode as a sign of undetected dementia among community dwelling subjects: a 2 year follow up study. J Neurol Neurosurg Psychiatry 2000, 69: 519-521. 10.1136/jnnp.69.4.519
Parthasarathy S, Tobin M: Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med 2002, 166: 1423-1429. 10.1164/rccm.200209-999OC
Alexopoulou C, Kondili E, Vakouti E, Klimathianaki M, Prinianakis G, Georgopoulos D: Sleep during proportional-assist ventilation with load-adjustable gain factors in critically ill patients. Intensive Care Med 2007, 33: 1139-1147. 10.1007/s00134-007-0630-2
Tung A, Lynch JP, Mendelson WB: Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesth Analg 2001, 92: 1232-1236. 10.1097/00000539-200105000-00028
Tung A, Bergmann BM, Herrera S, Cao D, Mendelson WB: Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology 2004, 100: 1419-1426. 10.1097/00000542-200406000-00014
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
About this article
Cite this article
Weinhouse, G.L., Schwab, R.J., Watson, P.L. et al. Bench-to-bedside review: Delirium in ICU patients - importance of sleep deprivation. Crit Care 13, 234 (2009). https://doi.org/10.1186/cc8131
Published:
DOI: https://doi.org/10.1186/cc8131