Abstract
Objective
To compare volume support ventilation (VSV) in which the pressure support level is continuously adjusted to deliver a preset tidal volume, with pressure support ventilation (PSV), in terms of patient behavior and ventilator response when ventilatory demand was increased by addition of dead space to the circuit.
Design and setting
Randomized cross-over study in an intensive care unit university hospital.
Interventions
We assessed in ten patients being weaned off mechanical ventilation the effect of increasing the ventilatory demand by adding a heat-and-moisture exchanger to augment the dead space with a fixed level of PSV and VSV.
Measurements and results
Arterial blood gases, breathing pattern, and respiratory effort parameters at the end of each of the four steps. Adding dead space significantly increased minute ventilation and PaCO2 values with both PSV and VSV. Indexes of respiratory effort (pressure-time index of respiratory muscles and work of breathing) increased with both ventilatory modes after dead-space augmentation. This increase was 2.5–4 times with VSV than with PSV and induced overt respiratory distress in two patients. The assistance delivered during VSV decreased significantly after dead-space augmentation, from 15.0±6.5 to 9.1±4.8 cmH2O, whereas no change occurred with PSV.
Conclusions
With a fixed level of VSV, but not of PSV, an increase in ventilatory demand results in a decrease in the pressure support provided by the ventilator, opposite to the desired response. VSV may conceivably result in respiratory distress in clinical settings.
Similar content being viewed by others
Introduction
Pressure-targeted modes have a number of limitations related to their dependency on respiratory mechanics. New ventilatory modes have been designed to offer a better response of the ventilator to changes in respiratory mechanics [1, 2]. Indeed, an increase in resistance or elastance during pressure support normally leads to a drop in tidal volume if no compensation occurs, from the patient or from the ventilator. These new modes, often called dual-control modes, use closed-loop feedback control systems that adapt the ventilator output based on the difference between the measured results of ventilation and a predefined target [1, 2, 3]. To overcome the theoretical limitations of pressure support ventilation volume-support ventilation (VSV) was introduced in the 1990s with the Siemens Servo 300 ventilator (Siemens Elema, Solna, Sweden). VSV is a pressure-limited mode that uses a target tidal volume and minute ventilation for feedback control. Thus the level of pressure support is continuously adjusted to deliver the preset VT. Two anecdotal reports with VSV [4, 5] and one randomized controlled trial [6] have shown variable results. Similar modes of ventilation working on the same principle-volume targeted pressure-regulated mode are now widely proposed by manufacturers on many of the new ventilators, without extensive clinical validation. No physiological studies, however, have specifically checked the efficacy of VSV in terms of adjustment to spontaneous changes in mechanics. Of greater concern, no study had looked at the response of such modes in the case of changes in ventilatory demand, such as those occurring for different states of wakefulness, nutrition, episodes of sepsis, pain, etc.
The aim of the present study was to compare VSV to PSV in terms of patient behavior and ventilator response when ventilatory demand was increased by addition of dead space to the circuit. The primary evaluation criteria were effort to breathe, as assessed by the respiratory muscle pressure-time index, work of breathing, and gas exchange. This work has been presented previously in abstract format [3].
Material and methods
Patients
Ten patients were prospectively enrolled who had been on mechanical ventilation via an endotracheal tube for more than 48 h and were ventilated with PSV levels of 10–20 cmH2O above positive end-expiratory pressure (PEEP). Exclusion criteria were as follows: coma or need for sedation, contraindication to gastric catheter insertion, hemoglobin level below 8 g/dl, body temperature above 38.5°C, and poor tolerance of PSV defined either as a respiratory rate greater than 30 breaths per minute and tidal volume lower than 6 ml/kg or use of the accessory inspiratory muscles. Patients’ characteristics are listed in Table 1 The experimental protocol was approved by the Ethics Committee of Saint-Eloi Teaching Hospital, Montpellier, and written informed consent was provided by each of the patients.
Protocol
During VSV the ventilator is designed to automatically adapt the pressure support level in response to changes in thoracopulmonary mechanical properties and in the patient’s inspiratory effort by comparing the observed tidal volume to the tidal volume target selected by the physician, taking into account a minimal calculated minute ventilation [2, 4, 5, 6]. As with PSV, the patient controls the respiratory rate, inspiratory time, and flow. The ventilator initiates VSV by delivering a test breath when patient effort is detected, with a peak pressure of 5 cmH2O (first-generation Siemens Servo 300 ventilator) or 10 cmH2O (second generation Siemens Servo 300 ventilator). The delivered tidal volume is measured and the ventilator makes a rough calculation of the impedance of the respiratory system over the first cycles. The ventilator then applies enough pressure to achieve the target tidal volume on a breath-by-breath basis. Once the target tidal volume is reached, the ventilator’s response to a rise in impedance is an increase in the pressure support level until the actual tidal volume matches the target tidal volume. Conversely, when the patient’s impedance decreases (i.e., when tidal volume becomes higher for a given level of pressure support), the ventilator reduces the level of pressure support. From breath-to-breath, the maximum pressure change is 3 cmH2O and can range from 0 cmH2O above PEEP to 5 cmH2O below the high-pressure alarm setting [1, 5, 6].
With VSV the respiratory rate must be set and serves three functions.
1. Based on the preset tidal volume the ventilator uses the preset respiratory rate to compute the “minimum minute ventilation” (respiratory rate multiplied by tidal volume). If the patient’s respiratory rate is lower than the preset respiratory rate, the minimum minute ventilation cannot be reached. The ventilator then computes a new target tidal volume as the reference for regulating pressure support and increases the applied pressure until this higher target tidal volume is reached in order to achieve the target minimum minute ventilation. The new tidal volume may be up to 150% of the preset tidal volume. For example, with a preset tidal volume of 600 ml and a preset respiratory rate of 10 breaths per minute the minimum minute ventilation is 6 l/min. If the respiratory rate drops below 10 breaths per minute, the maximum new target tidal volume is 900 ml.
2. The respiratory rate defines the respiratory cycle time. When the inspiration time exceeds 80% of the total set respiratory cycle time, the ventilator terminates the inspiration. The Siemens Servo 300 ventilator uses this as a secondary mechanism for terminating inspiration when the main criterion, i.e., and inspiratory flow decline to 5% of peak inspiratory flow, is not reached.
3. The respiratory rate serves as the ventilator rate if the patient becomes apneic, which activates the backup controlled mode.
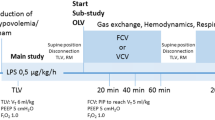
The effect of an increase in ventilatory demand, obtained by adding dead space to the circuit, was assessed with a fixed level of PSV and with VSV, in random order. The patients were studied in a comfortable semirecumbent position. With both modes the following sequence was applied in the same order: after a 20 min period of stable ventilation (“baseline”) a heat-and-moisture exchanger representing a dead space of 100 ml [7, 8, 9] was inserted between the tracheal tube and the Y-piece of the ventilatory circuit and left for 20 min (dead space period). Between the two modes 15 min of assist-control ventilation was used. Patients were not informed of the details of the changes during the two test periods.
Prior to the study period PSV was applied for 5 min to allow determination of the inspiratory pressure level that achieved a tidal volume between 6 and 8 ml/kg with a respiratory rate between 20 and 30 breaths per minute; the resulting values of pressure and tidal volume were used for PSV and VSV, respectively, and were applied throughout the study. With VSV the minimal respiratory rate was set at 10 breaths per minute. The extrinsic PEEP level was set at 4–6 cmH2O and kept constant throughout the study. FIO2 was set to achieve oxygen saturation greater than 95%.
Measurements
All measurements were performed during the last 5 min of the four study periods. The air flow rate was measured using a heated and calibrated pneumotachograph (Fleisch no. 1, Fleisch, Lausanne, Switzerland) that was linear over the experimental flow range. Airway pressure (Paw) was measured close to the pneumotachograph using a differential pressure transducer (MP45, ±100 cmH2O, Validyne, Northridge, Calif., USA). Esophageal and gastric pressures (Pes and Pga, respectively) were measured using a double-balloon catheter (Marquat, Boissy-Saint-Léger, France) inserted through a nostril after topical anesthesia then advanced until the distal and proximal balloons were in the stomach and midesophagus, respectively. Each balloon was filled with 0.5–1 ml air and connected to its own differential pressure transducer (MP45, ±100 cmH2O, Validyne). Placement of the esophageal balloon was assessed using the airway occlusion test [10] and placement of the gastric balloon by checking that smooth manual pressure on the abdomen produced Pga fluctuations and that swallowing produced a sharp Pes peak related to esophageal contraction with no Pga peak [11]. Transdiaphragmatic pressure (Pdi) was obtained by subtracting the Pes signal from the Pga signal (Pdi=Pga−Pes). Flow and pressure signals were digitized at 128 Hz and sampled using an analog-to-digital converter system (MP100, BIOPAC Systems, Santa Barbara, Calif., USA). After elimination of artifact cycles caused by coughing or esophageal or gastric spasms 15–20 consecutive breaths were used to compute mean values. Tidal volume was computed by integration of the flow signal and respiratory rate, inspiratory and expiratory times, duty cycle, and mean inspiratory flow rate were determined from the flow signal. Minute ventilation was calculated as the product of tidal volume by respiratory rate.
Data analysis and assessment of patient’s effort
The patient’s inspiratory effort was evaluated based on pressure indexes and inspiratory work of breathing (WOB). We measured the esophageal and transdiaphragmatic pressure swings (ΔPes and ΔPdi, respectively) and calculated the esophageal pressure-time product (PTPeso) and the transdiaphragmatic pressure-time product (PTPdi). The esophageal pressure-time product per cycle (PTPeso/br) was obtained by measuring the area under the Pes signal between the onset and end of inspiration and was normalized for the chest wall static recoil pressure-time relationship [12, 13]. The mean PTPeso/br (cmH2O s−1) value was multiplied by respiratory rate to obtain PTPeso/min (cmH2O s−1 min−1). PTPdi was calculated as the integral of transdiaphragmatic pressure over time during the phase of inspiratory muscular effort [12]. PTPdi per breath (PTPdi/br) was calculated as the area under the Pdi signal from the onset of its positive deflection to its return to baseline [14]. The mean PTPdi/br (cmH2O s−1) value was multiplied by RR to obtain PTPdi/min (cmH2O s−1 min−1).
Inspiratory WOB was computed from Pes and tidal volume loops as previously described [13, 15]. The inspiratory WOB per breath was calculated from a Campbell diagram by computing the area enclosed between the inspiratory esophageal pressure-tidal volume curve and the static esophageal pressure-volume curve of the chest wall, using a theoretical value for chest wall compliance (4% of the predicted value for the vital capacity per cmH2O). Although the use of this theoretical value may result in some error, this error would be identical for all periods and therefore would not affect the validity of comparisons. Inspiratory WOB is expressed as joules per breath, as the work per volume unit (joules per liter, or as the work per time unit (joules per minute). We hypothesized that no change in the mechanical properties of the respiratory system occurred during the study period.
Blood samples were obtained for arterial blood gas analysis (ABL 520 analyzer; Radiometer, Copenhagen) at the time of respiratory measurements through a 20-G catheter inserted in a radial or femoral artery. Standard three-lead monitoring electrodes continuously recorded heart rate and rhythm. Systolic and diastolic arterial blood pressures were continuously monitored through the radial artery signal. Oxygen saturation was continuously monitored using pulse oxymetry.
Statistical analysis
Values are expressed as mean ±standard deviation. The comparisons performed in the study were as follows: (a) baseline, VSV vs. PSV; (b) during VSV, baseline vs. dead space; (c) during PSV, baseline vs. dead space, and (d) delta (baseline–dead space), VSV vs. PSV. Nonparametric analysis of variance (Friedman’s test) and the Wilcoxon rank sum test were used for comparisons. Stat-view 5.0 software (SAS Institute) was employed. Differences at the level of p<0.05 were considered statistically significant.
Results
The mean values of the main ventilatory parameters are presented in Table 2 and blood gas and hemodynamic parameters in Table 3. None of the study parameters (ventilatory pattern, inspiratory effort, and hemodynamics) differed significantly between PSV and VSV during the baseline period (Tables 2, 3, 4). With PSV, tolerance was good during the baseline and dead-space periods in all patients. With VSV, however, two patients (nos. 2, 7) experienced overt clinical respiratory distress during the dead-space period, which had to be stopped prematurely. The data for the dead-space VSV period in these two patients were therefore recorded for about 5 min after addition of dead space.
Adding dead space significantly increased minute ventilation and PaCO2 values with both PSV and VSV (Tables 2, 3). Respiratory effort indexes at the end of the four studied conditions are summarized in Table 4 and individual values for WOB in Fig. 1. With both PSV and VSV all respiratory effort indexes increased after addition of dead space. With VSV the increases were about 2.5–4 times greater than with PSV, as shown in Fig. 2 for several indexes of inspiratory effort (ΔPdi, WOB, and PTPes). Recordings from an illustrative patient (no. 2) during the four conditions who had difficulty tolerating dead-space under VSV are shown in Fig. 3. Systolic and diastolic blood pressure increased significantly when dead space was applied with VSV, but not with PSV.
Increase in the main respiratory effort parameters: transdiaphragmatic pressure (Pdi), work of breathing (WOB, J/l) and pressure-time product of the respiratory muscles (PTPes, cmH2O s−1 min−1) with added dead space expressed in percentage of baseline value. Note that the increases with VSV were about 3–4 times those with PSV. *p<0.05
Experimental recordings in an illustrative patient (no. 2) during the four conditions, who had difficulty tolerating adding dead space under VSV. The esophageal (Pes) and transdiaphragmatic (Pdi) pressure swings were comparable at baseline for PSV and VSV, with a similar level of assist delivered by the ventilator. When dead space was applied with PSV, increases occurred in breathing rate and in esophageal and transdiaphragmatic pressure swings, but airway pressure remained unchanged. On the other hand, when dead space was applied with VSV, large increases were noted in breathing rate and in esophageal and transdiaphragmatic pressure swings, whereas the pressure-assist delivered by the ventilator decreased markedly
The assistance delivered by the ventilator decreased significantly after addition of dead-space with VSV, from 15.0±6.5 to 9.1±4.8 cmH2O (p<0.05); no change occurred with PSV. Figure 4 illustrates the changes in ΔPes swings and in the pressure support level delivered by the ventilator (Paw) immediately after addition of dead-space with VSV in a representative patient (no. 6). Pressure support decreased gradually after 2 min, from 15 to 8 cmH2O.
Changes in esophageal pressure swings (Pes), flow, tidal volume, and level of pressure assist delivered by the ventilator (Paw) recorded during VSV immediately after dead-space addition in a representative patient (no. 6). The pressure delivered by the ventilator decreased progressively after 2 min, from 15 cmH2O (point A on the airway-pressure waveform) to 8 cmH2O (point B on the airway-pressure waveform)
Discussion
The present study provides the first illustration of the response of a ventilator set on volume-guaranteed pressure-control mode to an increase in ventilatory demand compared with the same ventilator on pressure-support mode. In published studies of conventional PSV increasing the ventilatory demand by adding dead space was followed by increases in minute ventilation and inspiratory effort, without any change in the assistance delivered by the ventilator [16, 17, 18]. Our main finding is that an experimentally induced increase in ventilatory demand required far more effort on the part of the patient with VSV, as assessed by WOB and pressure-time product, than with PSV (Table 4, Figs. 1, and 2). This was due to the fact that the pressure level delivered by the ventilator decreased with VSV but remained unchanged with PSV in response to the increased ventilatory demand. The decrease with VSV was in direct contradiction to the desired response, which was provision of additional support. In other words, with VSV the more the patient worked, the less the ventilator assisted the patient (Figs. 3, 4). These results are somewhat expected from the algorithm of VSV, but have never been reported in clinical conditions in ICU patients.
Despite interest in dual-control modes such as VSV no published studies have evaluated patient-ventilator interactions in adults mechanically ventilated patients receiving VSV. Keenan and Martin [4] retrospectively reviewed their experience with 20 children treated with VSV for a total of 119 days. Of the 20 patients 12 were successfully extubated from VSV. Intolerance to VSV was noted in a single patient and due to an inconsistent respiratory drive. The authors concluded that VSV can be used successfully in a selected population of critically ill pediatric patients. There was no established protocol for VSV use, however, and no control group was available for comparison. Furthermore, the authors reported difficulty in choosing the initial ventilator settings and in determining when the patients were ready for extubation. Recently Sottiaux [5] reported that patient-ventilator asynchrony developing during PSV can be aggravated during VSV [19, 20]. Ineffective efforts are particularly frequent in patients with chronic obstructive pulmonary disease [21, 22]. In VSV mode, if the patient makes numerous ineffective efforts, the frequency of effective efforts recorded by the ventilator may be lower than the set frequency, in which case a new target tidal volume is calculated automatically to achieve the preset minute ventilation. This further increases the number of ineffective efforts and aggravates dysynchrony.
Randolph et al. [6] performed a controlled randomized trial in 182 infants and children who had been receiving ventilator support for more than 24 h. They evaluated whether a weaning protocol using VSV was superior over a weaning protocol using PSV or over standard care (i.e., no defined protocol). No differences occurred across the three groups for duration of weaning or management of sedative drugs. No complications were recorded in either the PSV or the VSV group. The authors found that, in contrast to adults, most infants and children were weaned off the ventilator within 2 days, and they noted no differences between the PSV and the VSV protocols [6].
Currently available closed-loop modes such as VSV and other dual modes use a single input to control a single output. To replicate the complexity of the respiratory system, which receives multiple inputs, a greater level of sophistication is needed [23, 24]. Although the one input-one output concept may generate appropriate ventilator responses to simple changes in respiratory system impedance, the present study illustrates clearly the limitations of this concept when changes in ventilatory demand occur. For instance, choosing low minute ventilation and tidal volume targets may lead to patient distress with tachypnea. When this occurs, the patient needs more assistance, yet according to our findings will receive less assistance. On the other hand, choosing high minute ventilation and tidal volume targets reduces arterial carbon dioxide tension, which may result in respiratory center depression. We chose to evaluate the effect of dead-space application instead of changes in respiratory elastance and/or resistance because it mimics a realistic increase in ventilatory demand which frequently occur in clinical practice (e.g., fever, pain, anxiety). However, the results of our study do not exclude that the behavior of VSV can differ substantially from that of PSV in other experimental settings, for example, mimicking clinical conditions characterized by an increase in resistance or elastance.
It may be interesting to compare VSV with proportional-assist ventilation (PAV), a pressure-assisted method of ventilatory support designed to provide inspiratory assistance in proportion to the instantaneous inspiratory muscle effort [25, 26]. PAV instantaneously adjusts the level of ventilator assistance to changes in the patient’s respiratory drive and workload [16, 17, 25, 26]. In this mode the ventilator generates pressure in proportion to both instantaneous flow and volume. In consequence the greater the inspiratory muscle effort, the higher is the Paw, and vice versa. Several studies [16, 17, 18, 25, 26, 27] have demonstrated that with PAV the respiratory loop gain increases significantly with increasing CO2 stimulus but remains constant with PSV and decreases with assist-controlled ventilation. We found that VSV did not function as a pressure-controlled mode when the ventilatory demand increased but instead resembled a volume-controlled mode. Indeed, as with assist-controlled ventilation, the respiratory loop gain decreased as respiratory effort increased [18, 27, 28].
In conclusion, our findings show that during VSV, a dual-control mode responsive to tidal volume, a ventilatory demand increase induced by addition of dead space leads to a decrease in pressure support, whereas no change occurs during standard PSV. The response to an added respiratory load required greater effort during VSV than during PSV. In clinical situations increased assistance from the ventilator should be the desired response of an adaptative mode to increased ventilatory demand (e.g., due to sepsis, fever, shivering, agitation, or pain).
References
Ranieri M (1997) Optimization of patient-ventilator interactions: closed-loop technology to turn the century. Intensive Care Med 23:936–939
Branson R, MacIntyre N (1996) Dual-control modes of mechanical ventilation. Respir Care 41:294–305
Jaber S, Delay J, Sebbane M, Matecki S, Chanques G, Eledjam J, Brochard L (2003) Evaluation d’un mode ventilatoire en pression asservi sur le volume (abstract). Reanimation Soins Intensifs Med Urgence SP171
Keenan H, Martin L (1997) Volume support ventilation in infants and children: analysis of a case series. Respir Care 42:281–287
Sottiaux TM (2001) Patient-ventilator interactions during volume-support ventilation: asynchrony and tidal volume instability-a report of three cases. Respir Care 46:255–262
Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, Luckett PM, Forbes P, Lilley M, Thompson J, Cheifetz IM, Hibberd P, Wetzel R, Cox PN, Arnold JH (2002) Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA 288:2561–2568
Pelosi P, Solca M, Ravagnan I, Tubiolo D, Ferrario L, Gattinoni L (1996) Effects of heat and moisture exchangers on minute ventilation, ventilatory drive, and work of breathing during pressure-support ventilation in acute respiratory failure. Crit Care Med 24:1184–1188
Jaber S, Chanques G, Matecki S, Ramonatxo M, Perrigault P, Souche B, Eledjam J (2002) Comparison of the effects of heat and moisture exchangers and heated humidifiers on ventilation and gas exchange during noninvasive ventilation. Intensive Care Med 28:1590–1594
Lellouche F, Maggiore SM, Deye N, Taille S, Pigeot J, Harf A, Brochard L (2002) Effect of the humidification device on the work of breathing during noninvasive ventilation. Intensive Care Medicine 28:1582–1589
Baydur A, Behrakis P, Zin W, Jaeger M, Milic-Emili J (1982) A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126:788–791
Diehl JL, Lofaso F, Deleuze P, Similowski T, Lemaire F, Brochard L (1994) Clinically relevant diaphragmatic dysfunction after cardiac operations. J Thorac Cardiovasc Surg 107:487–498
Sassoon C, Light R, Lodia R, Sieck G, Mahutte C (1991) Pressure-time product during continuous positive airway pressure, pressure support ventilation and T-piece during weaning from mechanical ventilation. Am Rev Respir Dis 143:459–475
Brochard L, Harf A, Lorino H, Lemaire F (1989) Inspiratory pressure support prevents diaphragmatic fatigue during weaning from mechanical ventilation. Am Rev Respir Dis 139:513–521
Barnard PA, Levine S (1986) Critique on application of diaphragmatic time tension index to spontaneously breathing humans. J Appl Physiol 54:1067–1072
Jaber S, Fodil R, Carlucci A, Boussarsar M, Pigeot J, Lemaire F, Harf A, Lofaso F, Isabey D, Brochard L (2000) Noninvasive ventilation with helium-oxygen in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161:1191–1200
Ranieri V, Giuliani R, Mascia L, Grasso S, Petruzelli V, Puntillo N, Perchiazzi G, Fiore T, Brienza A (1996) Patient-ventilator interaction during acute hypercapnia: pressure support vs. proportional assist ventilation. J Appl Physiol 81:426–436
Grasso S, Puntillo F, Mascia L, Ancona G, Fiore T, Bruno F, Slutsky AS, Ranieri VM (2000) Compensation for increase in respiratory workload during mechanical ventilation. Pressure-support versus proportional-assist ventilation. Am J Respir Crit Care Med 161:819–826
Chiumello D, Pelosi P, Calvi E, Bigatello L, Gattinoni L (2002) Different modes of assisted ventilation in patients with acute respiratory failure. Eur Respir J 20:925–933
Tobin M, Jubran A, Laghi F (2001) Patient-ventilator interaction. Am J Respir Crit Care Med 163:1059–1063
Brochard L (2002) When ventilator and patient’s end of inspiration don’t coincide; what’s the matter? Am J Respir Crit Care Med 166:2–3
Jubran A, Van de Graaff WB, Tobin MJ (1995) Variability of patient-ventilator interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 152:129–136
Nava S, Bruschi C, Rubini F, Palo A, Iotti G, Braschi A (1995) Respiratory response and inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med 21:871–879
Dojat M, Harf A, Touchard D, Laforest M, Lemaire F, Brochard L (1996) Evaluation of a knowledge-based system providing ventilatory management and decision for extubation. Am J Respir Crit Care Med 153:997–1004
Dojat M, Harf A, Touchard D, Lemaire F, Brochard L (2000) Clinical evaluation of a computer-controlled pressure support mode. Am J Respir Crit Care Med 161:1161–1166
Younes M (1992) Proportional assist ventilation, a new approach to ventilatory support. I. Theory. Am Rev Respir Dis 145:114–120
Younes M, Puddy A, Roberts D, Light RB, Quesada A, Taylor K, Oppenheimer L, Cramp H (1992) Proportional assist ventilation. Results of an initial clinical trial. Am Rev Respir Dis 145:121–129
Mitrouska J, Xirouchaki N, Patakas D, Siafakas N, Georgopoulos D (1999) Effects of chemical feedback on respiratory motor and ventilatory output during different modes of assisted mechanical ventilation. Eur Respir J 13:873–882
Georgopoulos D, Mitrouska I, Webster K, Bshouty Z, Younes M (1997) Effect of inspiratory muscle unloading on the response of respiratory motor output to CO2. Am J Respir Crit Care Med 155:2000–2009
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Jaber, S., Delay, JM., Matecki, S. et al. Volume-guaranteed pressure-support ventilation facing acute changes in ventilatory demand. Intensive Care Med 31, 1181–1188 (2005). https://doi.org/10.1007/s00134-005-2726-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2726-x