Abstract
Key message
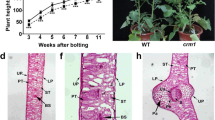
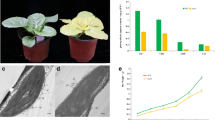
The cucumber chlorophyll-deficient golden leaf mutation is due to a single nucleotide substitution in the CsChlI gene for magnesium chelatase I subunit which plays important roles in the chlorophyll biosynthesis pathway.
Abstract
The Mg-chelatase catalyzes the insertion of Mg2+ into the protoporphyrin IX in the chlorophyll biosynthesis pathway, which is a protein complex encompassing three subunits CHLI, CHLD, and CHLH. Chlorophyll-deficient mutations in genes encoding the three subunits have played important roles in understanding the structure, function and regulation of this important enzyme. In an EMS mutagenesis population, we identified a chlorophyll-deficient mutant C528 with golden leaf color throughout its development which was viable and able to set fruits and seeds. Segregation analysis in multiple populations indicated that this leaf color mutation was recessively inherited and the green color showed complete dominance over golden color. Map-based cloning identified CsChlI as the candidate gene for this mutation which encoded the CHLI subunit of cucumber Mg-chelatase. The 1757-bp CsChlI gene had three exons and a single nucleotide change (G to A) in its third exon resulted in an amino acid substitution (G269R) and the golden leaf color in C528. This mutation occurred in the highly conserved nucleotide-binding domain of the CHLI protein in which chlorophyll-deficient mutations have been frequently identified. The mutant phenotype, CsChlI expression pattern and the mutated residue in the CHLI protein suggested the mutant allele in C528 is unique among mutations identified so far in different species. This golden leaf mutant not only has its potential in cucumber breeding, but also provides a useful tool in understanding the CHLI function and its regulation in the chlorophyll biosynthesis pathway as well as chloroplast development.





Similar content being viewed by others
References
Abul-Hayja Z, Williams PH (1976) Inheritance of two seedling markers in cucumber. Hort Sci 11:145
Braumann I, Stein N, Hansson M (2014) Reduced chlorophyll biosynthesis in heterozygous barley magnesium chelatase mutants. Plant Physiol Biochem 78:10–14
Campbell BW, Mani D, Curtin SJ, Slattery RA, Michno JM, Ort DR, Schaus PJ, Palmer RG, Orf JH, Stupar RM (2015) Identical substitutions in magnesium chelatase paralogs result in chlorophyll-deficient soybean mutants. G3 5:123–131
Cavagnaro PF, Senalik DA, Yang LM, Simon PW, Harkins TT, Kodira CD, Huang SW, Weng Y (2010) Genome-wide characterization of simple sequence repeats in cucumber (Cucumis sativus L.). BMC Genom 11:569
Deng XJ, Zhang HQ, Wang Y, Shu ZF, Wang GH et al (2012) Research advances on rice leaf-color mutant genes. Hybrid Rice 27:9–14 (in Chinese)
Deng XJ, Zhang HQ, Wang Y, He F, Liu JL, Xiao X, Wang GL (2014) Mapped clone and functional analysis of leaf-color gene ygl7 in a rice hybrid (Oryza sativa L. ssp. indica). PLoS One 9:e99564
Dong H, Fei GL, Wu CY, Wu FQ, Sun YY, Chen MJ, Ren YL, Zhou KN, Cheng ZJ, Wang JL, Jiang L, Zhang X, Guo XP, Lei CL, Su N, Wang H, Wan JM (2013) A rice virescent-yellow leaf mutant reveals new insights into the role and assembly of plastid caseinolytic protease in higher plants. Plant Physiol 162:1867–1880
Falbel TG, Meehl JB, Staehelin JA (1996) Severity of mutant phenotype in a series of chlorophyll-deficient wheat mutants depends on light intensity and the severity of the block in chlorophyll synthesis. Plant Physiol 112:821–832
Fodje MN, Hansson A, Hansson M, Olsen JG, Gough S, Willows RD, Al-Karadaghi S (2001) Interplay between an AAA module and an integrin I domain may regulate the function of magnesium chelatase. J Mol Biol 311:111–122
Gibson LC, Willows RD, Kannangara CG, von Wettstein D, Hunter CN (1995) Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: Reconstitution of activity by combining the products of the bchH, -I, and –D genes expressed in Escherichia coli. Proc Natl Acad Sci USA 92:1941–1944
Guo YM, Gu XF, Zhang CZ, Fang XJ, Zhang SP, Xu CQ (2003) Genetic mechanism of the cucumber leaf mutant. Acta Hort Sin 30:409–412 (in Chinese)
Hansson A, Kannangara CG, von Wettstein D, Hansson M (1999) Molecular basis for semi-dominance of missense mutations in the XANTHA-H (42-kDa) subunit of magnesium chelatase. Proc Natl Acad Sci USA 96:1744–1749
Hansson A, Willows RD, Roberts TH, Hansson M (2002) Three semi-dominant barley mutants with single amino acid substitutions in the smallest magnesium chelatase subunit form defective AAA+ hexamers. Proc Natl Acad Sci USA 99:13944–13949
Huang YS, Li HM (2009) Arabidopsis CHLI2 can substitute for CHLI1. Plant Physiol 150:636–645
Ikegami A, Yoshimura N, Motohashi K, Takahashi S, Romano PG, Hisabori T, Takamiya K, Masuda T (2007) The CHLI1 subunit of Arabidopsis thaliana magnesium chelatase is a target protein of the chloroplast thioredoxin. J Biol Chem 282:19282–19291
Jensen PE, Willows RD, Petersen BL, Vothknecht UC, Stummann BM et al (1996) Structural genes for Mg-chelatase subunits in barley: Xantha-f, -g and -h. Mol Gen Genet 250:383–394
Jensen PE, Gibson LC, Hunter CN (1999) ATPase activity associated with the magnesium-protoporphyrin IX chelatase enzyme of SynechocystisPCC6803: evidence for ATP hydrolysis during Mg2+ insertion, and the MgATP-dependent interaction of the ChlI and ChlD subunits. Biochem J339:127–134
Jung KH, Hur J, Ryu CH, Choi Y, Chung YY et al (2003) Characterization of a rice chlorophyll deficient mutant using the T-DNA gene-trap system. Plant Cell Physiol 44:463–472
Kannangara CG, Vothknecht UC, Hansson M, von Wettstein D (1997) Magnesium chelatase: association with ribosomes and mutant complementation studies identify barley subunit Xantha-G as a functional counterpart of Rhodobacter subunit BchD. Mol Gen Genet 254:85–92
Kobayashi K, Mochizuki N, Yoshimura N, Motohashi K, Hisabori T et al (2008) Functional analysis of Arabidopsis thaliana isoforms of the Mg-chelatase CHLI subunit. Photochem Photobiol Sci 7:1188–1195
Kropat J, Oster U, Rudiger W, Beck CF (1997) Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc Natl Acad Sci USA 94:14168–14172
Kropat J, Oster U, Rudiger W, Beck CF (2000) Chloroplast signaling in the light induction of nuclear HSP70 genes requires the accumulation of chlorophyll precursors and their accessibility to cytoplasm/nucleus. Plant J 24:523–531
Larkin RM, Alonso JM, Ecker JR, Chory J (2003) GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 299:902–906
Li YH, Yang LM, Pathak M, Li DW, He XM, Weng Y (2011) Fine genetic mapping of cp: a recessive gene for compact (dwarf) plant architecture in cucumber, Cucumis sativus L. Theor Appl Genet 123:973–983
Li WQ, Gao B, Yang J, Chen P, Li YH (2015) Physiological characterization of a new yellow leaf mutant. Acta Agri Boreal Occident Sin 24:98–103 (in Chinese)
Li S, Pan YP, Wen CL, Li YH, Liu XF, Zhang XL, Behera TK, Xing GM, Weng Y (2016) Integrated analysis in bi-parental and natural populations reveals CsCLAVATA3(CsCLV3) underlying carpel number variations in cucumber. Theor Appl Genet 129:1007–1022
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Lundqvist J, Braumann I, Kurowska M, Müller AH, Hansson M (2013) Catalytic turnover triggers exchange of subunits of the magnesium chelatase AAA motor unit. J Biol Chem 288:24012–24019
Luo T, Luo S, Araújo WL, Schlicke H, Rothbart M, Yu J, Fan T, Fernie AR, Grimm B, Luo M (2013) Virus-induced gene silencing of pea CHLI and CHLD affects tetrapyrrole biosynthesis, chloroplast development and the primary metabolic network. Plant Physiol Biochem 65:17–26
Markwell J, Osterman JC (1992) Occurrence of temperature-sensitive phenotypic plasticity in chlorophyll-deficient mutants of Arabidopsis thaliana. Plant Physiol 98:392–394
Masuda T (2008) Recent overview of the Mg branch of the tetrapyrrole biosynthesis leading to chlorophylls. Photosynth Res 96:121–143
Miao H, Zhang SP, Wang XW, Zhang ZH, Li M et al (2011) A linkage map of cultivated cucumber (Cucumis sativus L.) with 248 microsatellite marker loci and seven genes for horticulturally important traits. Euphytica 182:167–176
Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J (2001) Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA 98:2053–2058
Mochizuki N, Tanaka R, Grimm B, Masuda T, Moulin M, Smith AG, Tanaka A, Terry MJ (2010) The cell biology of tetrapyrroles: a life and death struggle. Trends Plant Sci 15:488–948
Nakayama M, Masuda T, Sato N, Yamagata H, Bowler C, OhtaH Shioi Y, Takamiya K (1995) Cloning, subcellular localization and expression of CHLI, a subunit of magnesium-chelatase in soybean. Biochem Biophys Res Commun 215:422–428
Pan YP, Bo KL, Cheng ZH, Weng Y (2015) The loss-of-function GLABROUS 3 mutation in cucumber is due to LTR-retrotransposon insertion in a class IV HD-ZIP transcription factor gene CsGL3 that is epistatic over CsGL1. BMC Plant Biol 15:302
Papenbrock J, Pfundel E, Mock H-P, Grimm B (2000a) Decreased and increased expression of the subunit CHLI diminishes Mg-chelatase activity and reduces chlorophyll synthesis in transgenic tobacco plants. Plant J 22:155–164
Papenbrock J, Mock HP, Tanaka R, Kruse E, Grimm B (2000b) Role of magnesium chelatase activity in the early steps of the tetrapyrrole biosynthetic pathway. Plant Physiol 122:1161–1169
Petersen BL, Moller MG, Jensen PE, Henningsen KW (1999) Identification of the Xan-g gene and expression of the Mg-chelatase encoding genes Xan-f, -g and -h in mutant and wild type barley (Hordeum vulgare L.). Hereditas 131:165–170
Pierce LK, Wehner TC (1990) Review of genes and linkage groups in cucumber. Hort Sci 25:605–615
Qin DD, Dong J, Xu FC, Guo GG, Ge ST, XuQ XuYX, Li MF (2015) Characterization and fine mapping of a novel barley stage green-revertible albino gene (HvSGRA) by bulked segregant analysis based on SSR assay and specific length amplified fragment sequencing. BMC Genom 16:838
Rissler HM, Collakova E, DellaPenna D, Whelan J, Pogson BJ (2002) Chlorophyll biosynthesis. Expression of a second chl I gene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol 128:770–779
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sawers RJ, Viney J, Farmer PR, Bussey RR, Olsefski G et al (2006) The maize Oil yellow1 (Oy1) gene encodes the I subunit of magnesium chelatase. Plant Mol Biol 60:95–106
Soldatova O, Apchelimov A, Radukina N, Ezhova T, Shestakov S, Ziemann V, Hedtke B, Grimm B (2005) An Arabidopsis mutant that is resistant to the protoporphyrinogen oxidase inhibitor acifluorfen shows regulatory changes in tetrapyrrole biosynthesis. Mol Genet Genom 273:311–318
Stenbaek A, Jensen PE (2010) Redox regulation of chlorophyll biosynthesis. Phytochemistry 71:853–859
Strand A, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature 421:79–83
Sun XQ, Wang B, Xiao YH, Wan CM, Deng XJ et al (2011) Genetic analysis and fine mapping of gene ygl98 for yellow-green leaf of rice. Acta Agron Sin 37:991–997
Suzuki JY, Bollivar DW, Bauer CE (1997) Genetic analysis of chlorophyll biosynthesis. Ann Rev Genet 31:61–89
Tang YL, HuangJF Wang RC (2004) Change law of hyperspectral data in related with chlorophyll and carotenoid in rice at different developmental stages. Rice Sci 11:274–282
Tian XQ, Ling YH, Fang LK, Du P, Sang XC, Zhao FM, Li YF, Xie R, He GH (2013) Gene cloning and functional analysis of yellow green leaf3 (ygl3) gene during the whole-plant growth stage in rice. Genes Genom 35:87–93
Vale RD (2000) AAA proteins lords of the ring. J Cell Biol 150:F13–F19
Von Wettstein D, Kahn A, Nielsen OF, Gough S (1974) Genetic regulation of chlorophyll synthesis analyzed with mutants in barley. Science 184:800–802
Von Wettstein D, Gough S, Kannangara CG (1995) Chlorophyll biosynthesis. Plant Cell 7:1039–1057
Walker CJ, Willows RD (1997) Mechanism and regulation of Mg-chelatase. Biochem J 327:321–333
Wang P, Gao J, Wan C, Zhang F, Xu Z, Huang X, Sun X, Deng X (2010) Divinyl chlorophyll (ide) a can be converted to monovinyl chlorophyll (ide) a by a divinyl reductase in rice. Plant Physiol 153:994–1003
Wilson-Sánchez D, Rubio-Díaz S, Muñoz-Viana R, Pérez-Pérez JM, Jover-Gil S, Ponce MR, Micol JL (2014) Leaf phenomics: a systematic reverse genetic screen for Arabidopsis leaf mutants. Plant J 79:878–891
Wu ZM, Zhang X, He B, Diao LP, Sheng SL, Wang JL, Guo XP, Su N, Wang LF, Jiang L, Wang CM, Zhai HQ, Wan JM (2007) A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol 145:29–40
Yang LM, Koo DH, Li YH, Zhang XJ, Luan FS, Havey MJ, Jiang JM, Weng Y (2012) Chromosome rearrangements during domestication of cucumber as revealed by high-density genetic mapping and draft genome assembly. Plant J 71:895–906
Yang LM, Li DW, Li YH, Gu XF, Huang SW, Garcia-Mas J, Weng Y (2013) A 1,681-locus consensus genetic map of cultivated cucumber including 67 NB-LRR resistance gene homolog and ten gene loci. BMC Plant Biol 13:53
Zhang H, Li J, Yoo H, Yoo SC, Cho SH et al (2006) Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Mol Biol 62:325–337
Zhang H, Liu LL, Cai MH, Zhu SS, Zhao JY, Zheng TH, Xu XY, Zeng ZQ, Niu J, Jiang L, Chen SH, Wan JM (2015) A point mutation of magnesium chelatase OsCHLI gene dampens the interaction between CHLI and CHLD subunits in rice. Plant Mol Biol Rep 33:1975–1987
Acknowledgments
The authors thank Kristin Haider for technical help. MG’s work was supported by the National Natural Science Foundation of China (No. 31401891), the Natural Science Foundation of Heilongjiang Province (C201330) and Young Outstanding Faculty Support Program in General Higher Education Institutions of Heilongjiang Province (1155G65). The work in YL’s lab was supported by the National Natural Science Foundation of China (31171955 and 31471891). The research in YW’s lab was supported by US Department of Agriculture Specialty Crop Research Initiative Grant (Project Number 2011-51181-30661).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Additional information
Communicated by R. G.F. Visser.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, M., Hu, L., Li, Y. et al. The chlorophyll-deficient golden leaf mutation in cucumber is due to a single nucleotide substitution in CsChlI for magnesium chelatase I subunit. Theor Appl Genet 129, 1961–1973 (2016). https://doi.org/10.1007/s00122-016-2752-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2752-9