Abstract
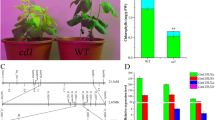
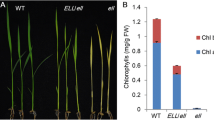
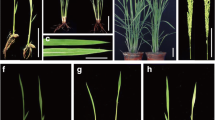
Photosynthetic organisms exhibit a green color due to the accumulation of chlorophyll pigments in chloroplasts. Mg-protoporphyrin IX chelatase (Mg-chelatase) comprises three subunits (ChlH, ChlD and ChlI) and catalyzes the insertion of Mg2+ into protoporphyrin IX, the last common intermediate precursor in both chlorophyll and heme biosyntheses, to produce Mg-protoporphyrin IX (MgProto). Chlorophyll deficiency in higher plants results in chlorina (yellowish-green) phenotype. To date, 10 chlorina (chl) mutants have been isolated in rice, but the corresponding genes have not yet been identified. Rice Chl1 and Chl9 genes were mapped to chromosome 3 and isolated by map-based cloning. A missense mutation occurred in a highly conserved amino acid of ChlD in the chl1 mutant and ChlI in the chl9 mutant. Ultrastructural analyses have revealed that the grana are poorly stacked, resulting in the underdevelopment of chloroplasts. In the seedlings fed with aminolevulinate-dipyridyl in darkness, MgProto levels in the chl1 and chl9 mutants decreased up to 25% and 31% of that in wild-type, respectively, indicating that the Mg-chelatase activity is significantly reduced, causing the eventual decrease in chlorophyll synthesis. Furthermore, Northern blot analysis indicated that the nuclear genes encoding the three subunits of Mg-chelatase and LhcpII in chl1 mutant are expressed about 2-fold higher than those in WT, but are not altered in the chl9 mutant. This result indicates that the ChlD subunit participates in negative feedback regulation of plastid-to-nucleus in the expression of nuclear genes encoding chloroplast proteins, but not the ChlI subunit.
Similar content being viewed by others
Abbreviations
- chl1 :
-
chlorina-1
- chl9 :
-
chlorina-9
- aa:
-
amino acid
- ChlH :
-
Mg-chelatase H subunit
- ChlI :
-
Mg-chelatase I subunit
- ChlD :
-
Mg-chelatase D subunit
- ALA:
-
5-aminolevulinate
- Proto:
-
protoporphyrin IX
- MgProto:
-
Mg-Protoporphyrin IX
- MgProtoME:
-
Mg-Protoporphyrin IX monomethyl ester
- MgProto(ME):
-
MgProto + MgProtoME
- DP:
-
2,2′-dipyridyl
References
Bellemare G, Bartlett SG, Chua N-H (1982) Biosynthesis of chlorophyll a/b-binding polypeptides in wild-type and the chlorina f2 mutant of barley. J Biol Chem 257:7762–7767
Biswal UC, Biswal B, Raval MK (2003) Protoplastid to chloroplast transformation. In: Chloroplast biogenesis. From proplastid to gerontoplast. Kluwer Academic Publishers, Dordrecht, pp 19–77
Confalonieri F, Duguet M (1995) A 200-amino acid ATPase module in search of a basic function. Bioassay 17:639–650
Dailey HA (1997) Enzymes of heme biosynthesis. J Biol Inorg Chem 2:411–417
Davison PA, Schubert HL, Reid JD, Iorg CD, Herou XA, Hill CP, Hunter CN (2005) Structural and biochemical characterization of Gun4 suggests a mechanism for its role in chlorophyll biosynthesis. Biochemistry 44:7603–7612
El-Shat HM (2000) Effects of s-aminolevulinic acid on pigment formation and chlorophyllase activity in French bean leaf. Acta Biol Hung 51:83–90
Falbel TG, Staehelin LA (1994) Characterization of a family of chlorophyll-deficient wheat (Triticum) and barley (Hordeum vulgare) mutants with defects in the magnesium-insertion step of chlorophyll biosynthesis. Plant Physiol 104:639–648
Fischerova H (1975) Linkage relationships of recessive chlorophyll mutations in Arabidopsis thaliana. Biol Plant 17:182–188
Fodje MN, Hansson A, Hansson M, Olsen JG, Gough S, Willows RD, Al-Karadaghi S (2001) Interplay between an AAA module and an integrin I domain may regulate the function of magnesium chelatase. J Mol Biol 311:111–122
Gibson LC, Willows RD, Kannangara CG, von Wettstein D, Hunter CN (1995) Magnesium-protoporphyrin chelatase of Rhodobacter sphaeroides: reconstitution of activity by combining the products of the BchH, -I, and -D genes expressed in Escherichia coli. Proc Natl Acad Sci USA 92:1941–1944
Grafe S, Saluz HP, Grimm B, Hanel F (1999) Mg-chelatase of tobacco: the role of the subunit CHL D in the chelation step of protoporphyrin IX. Proc Natl Acad Sci USA 96:1941–1946
Grimm B (1998) Novel insights into the control of tetrapyrrole metabolism of higher plants. Curr Opin Plant Biol 1:245–250
Hansson A, Kannangara CG, von Wettstein D, Hansson M (1999) Molecular basis for semidominance of missense mutations in the XANTH-H (42 kDa) subunit of magnesium chelatase. Proc Natl Acad Sci USA 96:1744–1749
Jensen PE, Gibson LC, Hunter CN (1998) Determinants of catalytic activity with the use of purified I, D and H subunits of the magnesium protoporphyrin IX chelatase from Synechocystis PCC6803. Biochem J 334:335–344
Jung KH, Hur J, Ryu CH, Choi Y, Chung YY, Miyao A, Hirochika H, An G (2003) Characterization of a rice chlorophyll-deficient mutant using the T-DNA gene-trap system. Plant Cell Physiol 44:463–472
Kannangara CG, Vothknecht UC, Hansson M, von Wettstein D (1997) Magnesium chelatase: association with ribosomes and mutant complementation studies identify barley subunit XANTHA-G as a functional counterpart of Rhodobacter subunit BchD. Mol Gen Genet 254:85–92
Kay BK, Williamson MP, Sudo M. (2000) The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J␣14:231–241
Kjemtrup S, Sampson KS, Peele CG, Nguyen LV, Conkling MA, Thompson WF, Robertson D (1998) Gene silencing from plant DNA carried by a Geminivirus. Plant J 14:91–100
Lake V, Olsson U, Willows RD, Hansson M (2004) ATPase activity of magnesium chelatase subunit I is required to maintain subunit D in vivo. Eur J Biochem 271:2182–2188
Larkin RM, Alonso JM, Ecker JR, Chory J (2003) GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 299:902–906
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Mochizuki N, Brusslan JA, Larkin R, Nagatani A, Chory J (2001) Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc Natl Acad Sci USA 98:2053–2058
Murray DL, Kohorn BD (1991) Chloroplasts of Arabidopsis thaliana homozygous for the ch-1 locus lack chlorophyll b, lack stable LHCPII and have stacked thylakoids. Plant Mol Biol 16:71–79
Nakayama M, Masuda T, Sato N, Yamagata H, Bowler C, Ohta H, Shioi Y, Takamiya K (1995) Cloning, subcellular localization and expression of CHLI, a subunit of magnesium-chelatase in soybean. Biochem Biophys Res Commun 215:422–428
Neuwald AF, Aravind L, Spouge JL, Koonin EV (1999) AAA+: a class of chaperone-like ATPase associated with the assembly, operation, and disassembly of protein complexes. Genome Res 9:27–43
Papenbrock J, Grimm B (2001) Regulatory network of tetrapyrrole biosynthesis – studies of intracellular signalling involved in metabolic and developmental control of plastids. Planta 213:667–681
Papenbrock J, Pfundel E, Mock H-P, Grimm B (2000) Decreased and increased expression of the subunit CHLI diminishes Mg chelatase activity and reduces chlorophyll synthesis in transgenic tobacco plants. Plant J 22:155–164
Rissler HM, Collakova E, DellaPenna D, Whelan J, Pogson BJ (2002) Chlorophyll biosynthesis. Expression of a second ChlI gene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol 128:770–779
Sawers RJH, Viney J, Farmer PR, Bussey RR, Olsefski G, Anufrikova K, Hunter CN, Brutnell TP (2006) The maize Oil Yellow 1 (Oy1) gene encodes the I subunit of magnesium chelatase. Plant Mol Biol 60:95–106
Soldatova O, Apchelimov A, Radukina N, Ezhova T, Shestakov S, Ziemann V, Hedtke B, Grimm B (2005) An Arabidopsis mutant that is resistant to the protoporphyrinogen oxidase inhibitor acifluorfen shows regulatory changes in tetrapyrrole biosynthesis. Mol Genet Genomics 273:311–318
Strand A, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421:79–83
Surpin M, Larkin R, Chory J (2002) Signal transduction between the chloroplast and the nucleus. Plant Cell 14:S327–338
Tripathy BC, Rebeiz CA (1985) Chloroplast biogenesis: quantitative determination of monovinyl and divinyl Mg-protoporphyrins and protochlorophyll(ides) by spectrofluorometry. Anal Biochem 149:43–61
von Wettstein D, Gough S, Kannangara CG (1995) Chlorophyll biosynthesis. Plant Cell 7:1039–1057
Walker CJ, Willows RD (1997) Mechanism and regulation of Mg-chelatase. Biochem J 327:321–333
Willows RD, Gibson LC, Kanangara CG, Hunter CN, von Wettstein D (1996) Three separate proteins constitute the magnesium chelatase of Rhodobacter sphaeroides. Eur J Biochem 235:438–443
Acknowledgements
We thank the Rice Genome Research Program (Japan) for providing cDNA stocks and the AIMS (USA) for T-DNA insertional mutant stocks. This research was supported by a grant (CG3131) from Crop Functional Genomics Center of the 21st century Frontier Research Program funded by the Ministry of Science and Technology (MOST) and Rural Development Administration (RDA) of Republic of Korea. Mr. H. Zhang was supported by a fellowship from the Korea Research Foundation Grant Funded by the Korea Government (MOEHRD) (KRF-2004-211-410039). Mr. J.-H. Yoo and S.-H.␣Cho were supported by Brain Korea 21 Project of Korean Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Haitao Zhang and Jinjie Li contributed equally to this work
Rights and permissions
About this article
Cite this article
Zhang, H., Li, J., Yoo, JH. et al. Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Mol Biol 62, 325–337 (2006). https://doi.org/10.1007/s11103-006-9024-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-006-9024-z