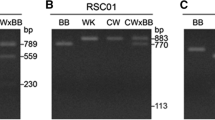
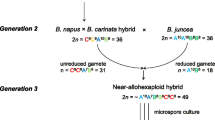
Abstract
Induced mutations were used to improve the low seed fertility of an intergeneric allopolyploid, ‘Baemoochae,’ ×Brassicoraphanus, synthesized following hybridization between Brassica rapa and Raphanus sativus. The mutagen N-methyl-N-nitroso-urethane (NMU) was added to microspore cultures. Four lines of nine in the Mi2 generation showed very high fertility under controlled pollination. The progeny lines (Mi3) confirmed this result under open pollination, and excellent uniformity was observed in plants grown in the field, as well as in their AFLP profile. On attaining high fertility and uniformity, one of the lines was released to farmers as a new leafy vegetable crop. The original nine lines shared very similar AFLP banding patterns, without any large differences between the high and low seed fertility lines. Thus, mutation induction accelerated genetic stabilization of a newly synthesized allopolyploid, ×Brassicoraphanus.


Similar content being viewed by others
References
Bang SW, Kaneko T, Matsuzawa Y, Bang KS (2002) Breeding of Moricandia arvensis monosomic chromosome addition lines (2n = 19) of alloplasmic (M. arvensis) Raphanus sativus. Breed Sci 52:193–199
Bannerot T, Boulidard L, cauderon Y, Tempe J (1974) Transfer of cytoplasmic male sterility from Raphanus sativus to Brassica oleracea. Eucarpia Meeting Cruciferae, Dundee, pp 52–54
Barro F, Fernandez-Escobar J, De La Vega M, Martin A (2001) Doubled haploid lines of Brassica carinata with modified erucic acid content through mutagenesis by EMS treatment of isolated microspores. Plant Breed 120:262–264
Chen ZJ, Pickard CS (1997) Transcriptional analysis of nucleolar dominance in polyploid plants: biased expression/silencing of progenitor rRNA genes is developmentally regulated in Brassica. Proc Natl Acad Sci USA 94:3442–3447
Chen HG, Wu JS (2008) Characterization of fertile amphidiploid between Rapanus sativus and Brassica alboglabra and the crossability with Brassica species. Genet Res Crop Evol 55:143–150
Dolstra O (1982) Synthesis and fertility of ×Brassicoraphanus and ways of transferring Raphanus characters to Brassica. Agric Res Rep 917:1–90
Gaeta RT, Pieres JC, Iniguez-Luy F, Leon E, Osborn TC (2007) Genomic changes in resynthesized Brassica napus and their effects on gene expression and phenotypic variation. Plant Cell 19:3403–3417
Gaeta RT, Yoo S-Y, Pieres JC, Doerge RW, Chen ZJ, Osborn TC (2009) Analysis of gene expression in resynthesized Brassica napus allopolyploids using Arabidopsis 70mer oligo mcroarrays. PLoS ONE 4:1–14
Ge X-H, Li Z-Y (2007) Intra- and intergenomic homology of B-genome chromosomes in trigenomic combinations of the cultivated Brassica species revealed by GISH analysis. Chromosom Res 15:849–861
Gómez-Campo C, Prakash S (1999) Origin and domestication. In: Gómez-Campo C (ed) Biology of Brassica coenospecies. Elsevier, Amsterdam, pp 33–58
Hong S-Y, Lee S-S (1995) Microspore culture of ×Brassicoraphanus. J Kor Soc Hort Sci 36:453–459
Howard HW (1938) The fertility of amphidiploids from the cross Raphanus sativus × Brassica oleracea. J Genet 36:239–273
Jenczewski E, Eber F, Grimaud A, Heut S, Lucas MO, Monod H, Chevre AM (2003) PrBn, a major gene controlling homoeologous pairing in oilseed rape (Brassica napus) haploids. Genetics 164:645–653
Jeong HW, Lee SS (1997) Influence of mutagen, n-nitroso n-methyl urethane on embryo induction and plant development in microspore culture of broccoli. J Korean Soc Hort Sci 38:379–383
Karpechenko GD (1927) Polyploid hybrid of Raphanus sativus L. x Brassica oleracea L. Bul Appl Bot Genet Plant Breed 7:305–410
Keller WA, Amstrong KC (1977) Embryogenesis and plant regeneration in Brassica napus anther culture. Can J Bot 55:1383–1388
Lange W, Toxopeus H, Lubberts JH, Dolstra O, Harrewijn JL (1989) The development raparadish (×Brassicoraphanus, 2n = 38), a new crop in agriculture. Euphytica 40:1–14
Lee SS, Woo JG, Shin HH (1989) Obtaining intergeneric hybrid plant between Brassica campestris and Raphanus sativus through young ovule culture. Korean J Breed 21:52–57
Lee S-S, Choi W-J, Woo J-G (2002) Development of a new vegetable crop in ×Brassicoraphanus by hybridization of Brassica campestris and Raphanus sativus. J Korean Soc Hort Sci 43:693–698
Lelivelt CLC, Lang W, Dolstra O (1993) Intergeneric crosses for the transfer of resistance to the beet cyst nematodes from Raphanus sativus to Brassica napus. Euphytica 68:111–120
Li Z-y, Ge X-h (2007) Unique chromosome behavior and genetic control in Brassica × Orychophragmus wide hybrids:a review. Plant Cell Rep 26:701–710
Lim SJ (2001) Confirmation of chromosomal constitution by means of karyotype analysis and genomic in situ hybridization of ×Brassicoraphanus, the intergeneric hybrid between Brassica campestris ssp. pekinensis and Raphnus sativus. MS Thesis Chung-Ang University pp 1–52
Lim S, Lee J, Kim J-K (2009) Analysis of isothiocyanates in newly generated vegetables, Baemuchae (×Brassicoraphanus) as affected by growth. Int J Food Sci Technol 44:1401–1407
Lukens LN, Pires JC, Leon E, Vogelzang R, Oslach L, Osborn T (2006) Patterns of sequence loss and cytosine methylation within a population of newly resynthesized Brassica napus allopolyploids. Plant Physiol 140:336–348
Ma N, Li Z-Y (2007) Development of novel Brassica napus lines with canola quality and higher levels of oleic and linoleic acids derived from intergeneric hybrids between B. napus and Orychophragmus violaceus. Euphytica 157:231–238
McClinchey SA, Kott LS (2008) Production of mutants with high cold tolerance in spring canola (Brassica napus). Euphytica 162:51–67
McNaughton IH (1979) The current position and problems in the breeding of Raphanobrassica (radicole) as a forage crop. In: Proceedings of the 4th Eucarpia-confererence Breed Cruciferous crops, pp 22–28
Murashige T, Skoog F (1968) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant 15:473–497
Namai H, Sarashima M, Hosoda T (1980) Interspecific and intergeneric hybridization breeding in Japan. In: Tsunoda S, Hinata K, Gomez-campo C (eds) Brassica crops and wild allies. Japan Sci Soc Press, Tokyo, pp 167–190
Olsson G, EllerstrÖm S (1980) Polyploidy breeding in Europe. In: Tsunoda S, Hinata K, Gomez-campo C (eds) Brassica crops and wild allies. Japan Sci Soc Press, Tokyo, pp 167–190
Peterka H, Budahn H, Schrader O, Ahne R, Schütze W (2004) Transfer of resistance against the beet cyst nematode from radish (Raphanus sativus) to rape (Brassica napus) by monosomic chromosome addition. Theor Appl Genet 109:30–41
Pires JC, Zhao J, Schranz ME, Leon EJ et al (2004) Flowering time divergence and genomic rearrangement in resynthesized Brassica polyploids (Brassicaceae). Biol J Linn Soc 82:675–688
Prakash S (2001) Utilization of wild germplasm of Brassica allies in developing male sterility-fertility restoration systems in Indian mustard-Brassica juncea. In: Houli L, Fu TD (eds) Proc Int Symp Rapeseed, Science Huazhong Agricultural Univ, China. Science Press, New York, pp 73–78
Prakash S, Bhat SR, Quiros CF, Kirti PB, Chopra VL (2009) Brassica and its close allies: cytogenetics and evolution. In: Janick Jules (ed) Plant Breed Rev, vol 31. John Wiley & Sons, Inc, London, pp 21–187
Primard C, Vedel FC, Matieu G, Pelletier G, chevre AM (1988) Interspecific somatic hybridization between Brassica napus and Brassica hirta (Sinapis alba L.). Theor Appl Genet 75:546–552
Rhee Y-H, Ahn K-S, Lee S-S, Park Y-D, Ryu S-Y, Kim S-H (2007) Isolation of chemical compounds from ×Brassicoraphanus. Korean J pharmacogn 38:403–408
Richharia RH (1937) Cytological investigations of Raphanus sativus, Brassica oleracea and their F1 hybrids. J Genet 34:45–55
Song KM, Osborn TC, Williams PH (1988) Brassica taxonomy based on nuclear restriction fragment length polymorphism (RFLP). 1. Genome evolution of diploid and amphidiploid species. Theor Appl Genet 75:784–794
Song KM, Lu P, Tang K, Osborn TC (1995) Rapid genome change in synthetic polyploids of Brassica and its implication for polyploid evolution. Proc Nat Acad Sci USA 92:7719–7723
Swanson EB, Herrgesell MJ, Arnoldo M, Sippell DW, Wong RSC (1989) Microspore mutagenesis and selection of canola plant with field tolerance to the imidazolinones. Theor Appl Genet 78:252–260
Tokumas S, Kato M (1988) Chromosomal and genic structure of Brassicoraphanus related to seed fertility and the presentation of an instance of improvement of its fertility. Euphytica 39:145–151
U N (1935) Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn J Bot 7:389–452
Vos P, Hogers R, Bleeke M, Reijans M, van de Lee T, Hornes M, Frijters A, pot Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Warwick SI, Black LD (1991) Molecular systematic of Brassica and allied genera (subtribe Brassicinae, Brassiceae)-chloroplast genome and cytodeme congruence. Theor Appl Genet 82:81–92
Xu Y, Zhong L, Wu X, Fang X, Wang J (2009) Rapid alterations of gene expression and cytocine methylation in newly synthesized Brassica napus allopolyploids. Planta 229:471–483
Zao ZG, Ma N, Li ZY (2007) Alteration of chromosome behavior and synchronization of parental chromosomes after successive generations in Brassica napus × Orychophragmus violaceus hybrids. Genome 50:226–233
Acknowledgments
This work was supported by the Institute of Planning and Evaluation for Technology, Ministry of Food, Agriculture, Forestry, and Fisheries of Korea (506017-05-SB010) and AgriFood New Material Research Center, Gyonggi Regional Research Center, Chung-Ang University. We thank Dr. Prakash, National Research Center on Plant Biotechnology, Indian Agricultural Research Institute, for reviewing and commenting on this manuscript. We would also like to thank the personnel at BBI for their assistance in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Becker.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, SS., Lee, SA., Yang, J. et al. Developing stable progenies of ×Brassicoraphanus, an intergeneric allopolyploid between Brassica rapa and Raphanus sativus, through induced mutation using microspore culture. Theor Appl Genet 122, 885–891 (2011). https://doi.org/10.1007/s00122-010-1494-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-010-1494-3